| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| Other Sizes |
| 靶点 |
RORγt; Retinoic acid receptor-related orphan nuclear receptor gamma t (RORγt) (IC50: 9.6 nM in competitive binding assays) [1]
|
|---|---|
| 体外研究 (In Vitro) |
在用表达 RORγt 的载体转染的 HEK-293 T 细胞中,JNJ-61803534 抑制 RORγt 转录,IC50 为 9.6 nM [1]。当暴露于 JNJ-61803534 (1 nM-1 μM) 时,源自人血液的 CD4+ T 细胞产生较少的 IL-17A、IL-17F、IFNγ 和 IL-22 [1]。体外Treg分化.
- IL-17抑制作用:JNJ-61803534在Th17分化条件下的人CD4+ T细胞中有效抑制IL-17A、IL-17F和IL-22的产生。ELISA分析显示剂量依赖性抑制,IL-17A的IC50为12 nM [1] - 选择性:在使用Gal4融合受体构建体转染的HEK-293T细胞的1-杂交报告基因实验中,该化合物对RORγt的选择性超过RORα和RORβ 100倍以上 [1] - 调节性T细胞保护:JNJ-61803534(最高1 μM)处理不影响体外调节性T细胞(Tregs)的Foxp3基因表达或抑制功能 [1] |
| 体内研究 (In Vivo) |
JNJ-61803534(100 mg/kg,口服)可抑制小鼠血液中体外刺激的 IL-17A 合成[1]。在胶原诱导性关节炎 (CIA) 小鼠模型中,JNJ-61803534(3–100 mg/kg BID 或 60 mg/kg QD,口服)可减少炎症、软骨退化和骨质流失[1]。在小鼠中,JNJ-61803534(30 和 100 mg/kg,口服)可减轻咪喹莫特诱导的皮肤银屑病样炎症[1]。
- 胶原诱导性关节炎(CIA)模型:口服JNJ-61803534(3–100 mg/kg/天)剂量依赖性地将小鼠临床关节炎评分降低40–90%。组织病理学分析显示滑膜炎症和软骨破坏显著减轻 [1] - 咪喹莫特(IMQ)诱导的皮肤炎症:每日口服JNJ-61803534(30–100 mg/kg)使小鼠皮肤病变严重程度降低50–70%,同时耳组织中IL-17A、IL-17F和IL-22的mRNA表达减少 [1] - 药效动力学响应:在小鼠PK/PD模型中,单次口服100 mg/kg的JNJ-61803534使全血中IL-17A的体外抑制率达到80%,活性持续超过72小时 [1] |
| 酶活实验 |
- RORγt配体结合实验:重组人RORγt配体结合域与浓度范围为0.1 nM至10 μM的JNJ-61803534孵育。通过荧光偏振法测量结合亲和力,剂量响应曲线拟合确定IC50为9.6 nM [1][2]
- 转录活性实验:用RORγt-Gal4融合蛋白和荧光素酶报告质粒转染的HEK-293T细胞,经JNJ-61803534(0.01–10 μM)处理。24小时后测量荧光素酶活性,显示剂量依赖性抑制RORγt介导的转录,EC50为15 nM [1] |
| 细胞实验 |
- Th17极化实验:人CD4+ T细胞用抗CD3/CD28磁珠激活,并在Th17条件(IL-6、IL-23、TGF-β)下极化。分化过程中加入JNJ-61803534(0.1–100 nM)以剂量依赖性方式减少IL-17A分泌,IC50为12 nM [1]
- 全血实验:人全血在佛波酯(PMA)和离子霉素刺激下加入JNJ-61803534(0.01–10 μM)。流式细胞术分析显示IL-17A+ CD4+ T细胞呈剂量依赖性抑制,50%抑制浓度为8 nM [1] |
| 动物实验 |
Animal/Disease Models: Mouse collagen-induced arthritis (CIA) model[1]
Doses: 3-100 mg/kg BID or 60 mg/kg QD Route of Administration: Oral administration (po) Experimental Results: diminished clinical arthritis scores and hind paw histopathology scores. - CIA Model: C57BL/6 mice were immunized with type II collagen. From day 21 post-immunization, JNJ-61803534 was administered orally at 3, 10, 30, or 100 mg/kg twice daily for 14 days. Clinical scores were assessed daily based on paw swelling and erythema [1] - IMQ Model: Female BALB/c mice received topical application of 5% IMQ cream on the back skin for 7 days. JNJ-61803534 was administered orally once daily (30–100 mg/kg) starting on day 1. Skin inflammation was scored daily for thickness, redness, and scaling [1] - PK/PD Model: CD-1 mice received a single oral dose of 100 mg/kg JNJ-61803534. Blood samples were collected at 0, 2, 4, 8, 24, 48, and 72 hours post-dose. Plasma concentrations were measured by LC-MS/MS, and ex vivo IL-17A inhibition was determined by ELISA [1] |
| 药代性质 (ADME/PK) |
- Half-life: In healthy human volunteers, JNJ-61803534 exhibited a median terminal elimination half-life of 164–170 hours after single oral doses up to 200 mg [1]
- Bioavailability: Oral bioavailability was estimated to be 75% based on plasma exposure (AUC) following oral vs. intravenous administration in preclinical species [1] - Protein Binding: The compound showed >99% plasma protein binding in human serum, primarily to albumin and α1-acid glycoprotein [1] - Metabolism: JNJ-61803534 is metabolized in the liver via oxidation and glucuronidation, with no major active metabolites identified [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
- Preclinical Safety: In 1-month repeated-dose toxicity studies in rats and dogs, JNJ-61803534 was well tolerated at doses up to 100 mg/kg/day, with no significant effects on hematology, clinical chemistry, or histopathology [1]
- Clinical Safety: In a phase 1 study, single ascending doses of JNJ-61803534 up to 200 mg were generally safe and well tolerated in healthy volunteers, with no serious adverse events reported. The most common treatment-related effects were mild headache and gastrointestinal symptoms [1] - Drug-Drug Interactions: JNJ-61803534 is a substrate of CYP3A4/5 and P-glycoprotein. Co-administration with strong CYP3A inhibitors (e.g., ketoconazole) increased plasma exposure by 2.5-fold, while inducers (e.g., rifampin) decreased exposure by 60% [1] |
| 参考文献 | |
| 其他信息 |
- Mechanism of Action: JNJ-61803534 acts as a reverse agonist of RORγt, blocking its interaction with co-activators and suppressing transcription of Th17-specific cytokines (IL-17A, IL-17F, IL-22) [1]
- Therapeutic Potential: The compound is being evaluated in phase 2 clinical trials for psoriasis, rheumatoid arthritis, and inflammatory bowel disease, with preliminary results showing dose-dependent improvements in disease activity [1] - Pharmacodynamic Biomarker: Ex vivo IL-17A inhibition in whole blood has been validated as a surrogate biomarker for target engagement and drug efficacy [1] |
| 分子式 |
C23H23CL2F6N3O4S
|
|---|---|
| 分子量 |
622.407843828201
|
| 精确质量 |
621.069
|
| 元素分析 |
C, 44.38; H, 3.72; Cl, 11.39; F, 18.31; N, 6.75; O, 10.28; S, 5.15
|
| CAS号 |
1917306-14-9
|
| PubChem CID |
121303394
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
5.3
|
| tPSA |
131
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
12
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
39
|
| 分子复杂度/Complexity |
913
|
| 定义原子立体中心数目 |
1
|
| SMILES |
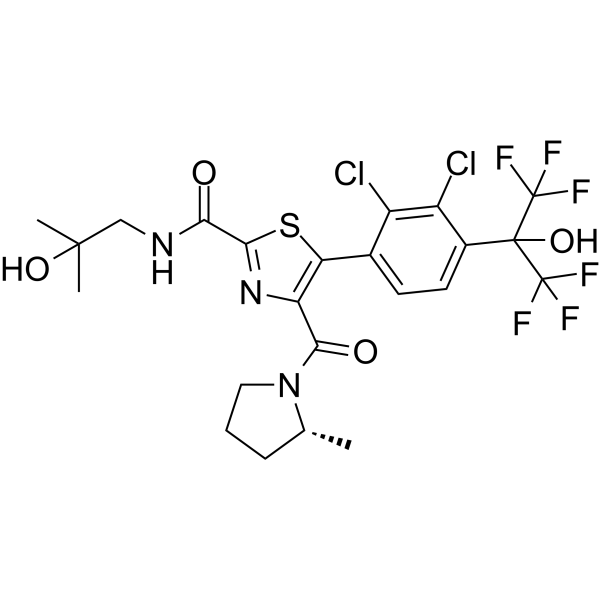
S1C(C2=CC=C(C(O)(C(F)(F)F)C(F)(F)F)C(Cl)=C2Cl)=C(C(N2CCC[C@H]2C)=O)N=C1C(NCC(O)(C)C)=O
|
| InChi Key |
ZQHVEGQHOKDZEN-SNVBAGLBSA-N
|
| InChi Code |
InChI=1S/C23H23Cl2F6N3O4S/c1-10-5-4-8-34(10)19(36)15-16(39-18(33-15)17(35)32-9-20(2,3)37)11-6-7-12(14(25)13(11)24)21(38,22(26,27)28)23(29,30)31/h6-7,10,37-38H,4-5,8-9H2,1-3H3,(H,32,35)/t10-/m1/s1
|
| 化学名 |
5-[2,3-dichloro-4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl]-N-(2-hydroxy-2-methylpropyl)-4-[(2R)-2-methylpyrrolidine-1-carbonyl]-1,3-thiazole-2-carboxamide
|
| 别名 |
JNJ-61803534; 1917306-14-9; US10150762, Example 2/16; CHEMBL5180983; SCHEMBL17711501; ZQHVEGQHOKDZEN-SNVBAGLBSA-N;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 300 mg/mL (482.00 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6067 mL | 8.0333 mL | 16.0666 mL | |
| 5 mM | 0.3213 mL | 1.6067 mL | 3.2133 mL | |
| 10 mM | 0.1607 mL | 0.8033 mL | 1.6067 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。