| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |

| 靶点 |
kappa-opioid receptor/KOR( Kd = 2.2 nM); μ-opioid receptor/MOR (Kd = 430 nM)
|
|---|---|
| 体外研究 (In Vitro) |
在急性感染的血液单核细胞衍生巨噬细胞 (MDM) 中,(-)-U-50488 盐酸盐(1 pM-100 nM;7 天)显示出 HIV-1 表达浓度依赖性降低,最大抑制发生在 10− 13 M(约 73% 抑制)[2]。 (-)-U-50488 盐酸盐(10-13 M;7-14 天)(10−13 M) 对 MDM 中的 HIV-1 感染具有长期抑制作用,并且在 7 和 14 天显着抑制 HIV-1 表达感染后的天数[3]。
U50488对急性感染MDM患者HIV-1表达的影响[3] 用U50488治疗MDM导致感染后第7天HIV-1表达的显著抑制(图1A)。U50488的抑制作用呈浓度依赖性,呈U形剂量-反应曲线,在10-13M时显示出最大抑制作用(约73%抑制)。为了进一步证实U50488的这种抗HIV-1作用,还检查了感染后14天收获的培养上清液。同样,U50488(10-13M)在感染后7天和14天显著抑制了HIV-1的表达(P<0.01)(图1B),表明U50488对MDM中的HIV-1感染具有持续的抑制作用。用10-12M Nor-BNI预处理小胶质细胞培养物,可拮抗U50488(10-13M)对HIV-1表达的抑制作用>65%(图2)(P<0.01),表明U50488的抗HIV-1作用是由KOR介导的。我们还发现,用U50488预处理MDM对其抗病毒作用不是必需的(在感染前6小时和24小时同时治疗的MDM中,p24-Ag产生的抑制率分别为65%、68%和64%)。 细胞因子或趋化因子的参与[3] 由于某些促炎细胞因子(即IL-1β、IL-6和TNF-α)和趋化因子RANTES具有强大的抗HIV-1活性(Herbein和Gordon,1997,Lokensgard等人,1997),接下来评估了U50488的抗HIV1作用是否涉及这些介体的释放。为了验证这一假设,在用U50488治疗之前,将针对这些分子的特异性抗体添加到MDM培养物中。虽然用1μg/ml的IL-1β、IL-6或TNF-α特异性抗体治疗急性感染的MDM未能阻断U50488的抗HIV-1作用,但用RANTES特异性抗体的治疗使U50488抗HIV-1的作用减弱了57±6%(图5)。这一发现表明,刺激RANTES的产生在U50488的抗HIV-1作用中起作用。 |
| 体内研究 (In Vivo) |
在伏隔核 (NAc) 中,(-)-U-50488 盐酸盐(腹膜内注射;5 mg/kg;4% 多聚甲醛 (PFA) 前 2 小时)选择性急性诱导 pMeCP2-S421(甲基 DNA 结合的磷酸化)蛋白质 MeCP2 (Ser421) 和 Fos,但没有改变大脑任何其他区域的 MeCP2 水平[2]。
U50488给药在NAc中急性诱导了pMeCP2-S421和Fos的选择性,但没有改变任何脑区的MeCP2水平。U50488诱导的CPA与ILC和BLA中pMeCP2-S421的减少以及BLA中Fos的诱导有关。MeCP2-KI小鼠显示CPA与WT同窝小鼠无法区分,但它们在CPA后也显示出较少的BLA-Fos诱导。 结论:这些数据首次表明,U50488给药后,pMeCP2-S421在脑中急性诱导,但在U50488诱导的CPA后没有。尽管U50488诱导的CPA不需要pMeCP2-S421,但这种磷酸化事件可能有助于控制厌恶行为的大脑区域的分子可塑性。[2] |
| 细胞实验 |
MDM的治疗和感染[3]
为了评估KOR激活对急性感染MDM中HIV-1表达的直接影响,我们首先用指定浓度的U50488预处理这些细胞24小时,然后以0.02的感染倍数感染HIV-1SF162 24小时,如前所述(Lokensgard等人,1997)。在广泛洗涤后,将U50488重新加入培养基中,并在感染后第7天或第14天收获上清液,用于HIV-1 p24抗原(Ag)水平的测定。如前所述(Chao等人,1996),使用酶联免疫吸附试验测量HIV-1 p24-Ag水平。该测定的灵敏度为30pg/ml。 |
| 动物实验 |
Animal/Disease Models: C57BL/6J mice[2]
Doses: 5 mg/kg Route of Administration: intraperitoneal (ip) injection; single dose; 2 hrs before 4% PFA Experimental Results: Induced pMeCP2-S421 in the brain acutely. Acute U50488 Exposure [2] C57BL6 male mice were placed in the open field for 30 min prior to injection of Vehicle (saline) or 5mg/kg i.p. U50488. 2 hrs later mice were transcardially perfused with 4% paraformaldehyde (PFA) and 0.1 M PBS. Brains were postfixed overnight in PBS with 4% PFA and then transferred to 20% sucrose in PBS with 4% PFA. Vehicle-treated (control) mice received saline in alternative chambers on each day. Drug-treated mice were treated with U50488 (5mg/kg i.p.) on days 2,4, and 6 in the baseline more preferred chamber and with saline on days 3,5, and 7 in the alternate chamber. Finally on day 8, the challenge day, all mice were placed in the center chamber and allowed free access to the entire apparatus for 30 min with no drug exposures. On challenge day, total time spent in each chamber was measured and preference scores were determined as above. By comparing the time spent in the two chambers (drug/saline) and how their difference changes during conditioning, the preference score captures the animal’s true change in place preference, irrespective for example of shifts in time spent in the neutral (gray) chamber of the apparatus. 2 hours following behavioral analysis on challenge day, mice were perfused for brain immunostaining or were killed with CO2 and brain regions were harvested for western blotting. [2] Objectives: The goal was to establish the role and regulation of pMeCP2-S421 in corticolimbic brain regions of mice upon acute treatment with the kappa opioid receptor agonist U50488 and during the expression of U50488-induced conditioned place aversion (CPA). Methods: pMeCP2-S421 levels were measured in the nucleus accumbens (NAc), prelimbic cortex, infralimbic cortex (ILC), and basolateral amygdala (BLA) of male mice after intraperitoneal administration of U50488 and upon the expression of U50488-induced CPA. Fos was measured as marker of neural activity in the same brain regions. U50488-induced CPA and Fos levels were compared between knockin (KI) mice that lack pMeCP2-S421 and their wild-type (WT) littermates. [2] |
| 参考文献 |
|
| 其他信息 |
Opioids may play an immunomodulatory role in the pathogenesis of human immunodeficiency virus-1 (HIV-1) infection. Recently, synthetic kappa-opioid receptor (KOR) ligands have been found to have anti-human immunodeficiency virus type 1 activity in acutely infected brain macrophages. In the present study, we investigated whether the selective KOR ligand U50488 would exert such an anti-HIV-1 effect in acutely infected blood monocyte-derived macrophages (MDM). Treatment of acutely infected MDM with U50488 induced a concentration-dependent inhibition of HIV-1 expression. The dose--response relationship of U50488 was U-shaped with a peak effect observed at 10(-13) M, which was evident at both 7 and 14 days post-infection. The KOR antagonist nor-binaltorphimine blocked the anti-HIV-1 effect of U50488 by 73%, indicating involvement of a KOR-mediated mechanism. Also, expression of KOR mRNA and binding activity with a fluorescence-labeled KOR ligand supported the existence of KOR on MDM. Antibodies to the beta-chemokine, RANTES (regulated on activation normal T-cell expressed and secreted), but not to various other cytokines, blocked U50488 inhibition by 56% suggesting that the anti-HIV-1 effect of U50488 involved, in part, the production of RANTES by MDM. Taken together, these in vitro findings support the anti-HIV-1 property of U50488, and suggest that KOR ligands may have therapeutic potential for treating patients with acquired immunodeficiency syndrome. [3]
Purpose: A detailed review on the chemistry and pharmacology of non-fentanil novel synthetic opioid receptor agonists, particularly N-substituted benzamides and acetamides (known colloquially as U-drugs) and 4-aminocyclohexanols, developed at the Upjohn Company in the 1970s and 1980s is presented. Method: Peer-reviewed literature, patents, professional literature, data from international early warning systems and drug user fora discussion threads have been used to track their emergence as substances of abuse. Results: In terms of impact on drug markets, prevalence and harm, the most significant compound of this class to date has been U-47700 (trans-3,4-dichloro-N-[2-(dimethylamino)cyclohexyl]-N-methylbenzamide), reported by users to give short-lasting euphoric effects and a desire to re-dose. Since U-47700 was internationally controlled in 2017, a range of related compounds with similar chemical structures, adapted from the original patented compounds, have appeared on the illicit drugs market. Interest in a structurally unrelated opioid developed by the Upjohn Company and now known as BDPC/bromadol appears to be increasing and should be closely monitored. Conclusions: International early warning systems are an essential part of tracking emerging psychoactive substances and allow responsive action to be taken to facilitate the gathering of relevant data for detailed risk assessments. Pre-emptive research on the most likely compounds to emerge next, so providing drug metabolism and pharmacokinetic data to ensure that new substances are detected early in toxicological samples is recommended. As these compounds are chiral compounds and stereochemistry has a large effect on their potency, it is recommended that detection methods consider the determination of configuration. [1] A detailed description of U-50488H was first published in the peer reviewed literature in 1982. It did not cause respiratory depression and was not habit-forming, but was found to cause sedation, diuresis and dysphoria, the latter properties being undesirable in medicinal products and indicative of KOR-mediated effects. The (−) trans-(1S,2S) U-50488 (U-50488H) and the (±) trans-racemic mixture are commercially available as reagents from specialist chemical supply companies (e.g. Merck, Tocris Bioscience, Axon Medchem etc.) and are widely used as model KOR agonists in experimental studies. The discovery and deconvolution of the structure activity relationships of U-50488H, and its related compounds, including the important role of stereochemistry in agonist activity, was elegantly described by the Upjohn chemist responsible for its development, Jacob Szmuszkovicz [15]. The generic structure of the N-substituted benzamides and acetamides is shown in Fig. 2a. The structural requirements for a potent KOR agonist appear to be a combination of the following: (1) a (−) trans-(1S,2S) configuration; (2) the presence of an N-methyl group on the nitrogen adjacent to the carbonyl and (3) the presence of a methylene (CH2) “spacer”. This is further illustrated by the modifications of the U-50488H structure which lead to the synthesis of two further highly selective KOR agonists with a (−) trans-(1S,2S) configuration; U-62066 (spiradoline, 2-(3,4-dichlorophenyl)-N-methyl-N-[(5R,7S,8S)-7-pyrrolidin-1-yl-1-oxaspiro[4.5]decan-8-yl]acetamide) and U-69593 (N-methyl-2-phenyl-N-[(5R,7S,8S)-7-pyrrolidin-1-yl-1-oxaspiro[4.5]decan-8-yl]acetamide) both of which have a heterocyclic ring structure fused to the cyclohexyl moiety in the R3 position (Fig. 2a, Table 1). The properties of U-50488H were compared to those of the assumed novel mu-opioid (MOR) agonists U-47109 (3,4-dichloro-N-(2-(dimethylamino)cyclohexyl)benzamide), U-47700 and U-51754 (2-(3,4-dichlorophenyl)-N-[2-(dimethylamino)cyclohexyl]-N-methyl-acetamide) (Fig. 2) to demonstrate the drug’s selectivity for the KOR. In this study, U-47700, which has a (+) trans-(1R,2R) configuration (and it is assumed U-47109 and U-51754 also have the same configuration), was found to have 7.5, eight and three times greater analgesic effect than morphine in antinociceptive tests (tail flick, tail pinch and hydrochloric acid writhing respectively). U-50488H, U-51754 and U-47109 were found to be less potent than morphine, but still had measurable analgesic effects. Characteristic MOR mediated effects (Straub tail, arched back, increased locomotor activity) were observed when mice were treated with U-47700, U-51754 and U-47109 but were not observed for U-50488H. [1] |
| 分子式 |
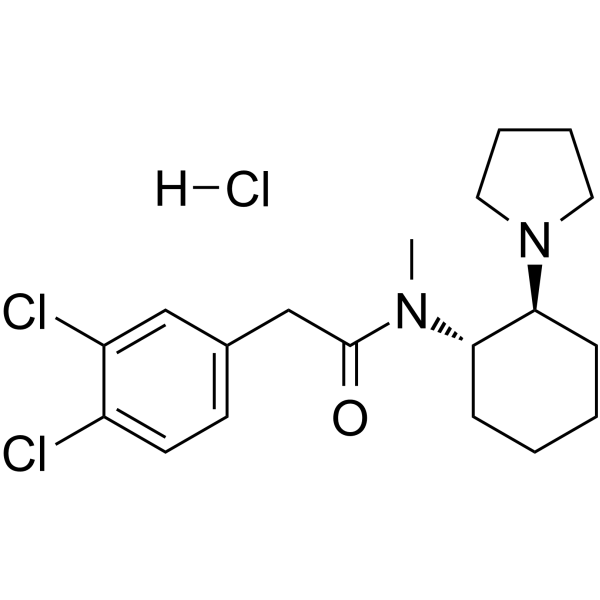
C19H27CL3N2O
|
|---|---|
| 分子量 |
405.79
|
| 精确质量 |
422.129
|
| CAS号 |
114528-79-9
|
| 相关CAS号 |
(1R,2R)-U-50488 hydrochloride;109620-49-7;(±)-U-50488 hydrochloride;67197-96-0;(±)-U-50488 hydrate hydrochloride;(+)-U-50488;67198-17-8;(+)-U-50488 hydrochloride;114528-81-3
|
| PubChem CID |
9931141
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
5.076
|
| tPSA |
32.78
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
25
|
| 分子复杂度/Complexity |
428
|
| 定义原子立体中心数目 |
2
|
| SMILES |
CN([C@H]1CCCC[C@@H]1N2CCCC2)C(=O)CC3=CC(=C(C=C3)Cl)Cl.Cl
|
| InChi Key |
KGMMGVIYOHGOKQ-APTPAJQOSA-N
|
| InChi Code |
InChI=1S/C19H26Cl2N2O.ClH/c1-22(19(24)13-14-8-9-15(20)16(21)12-14)17-6-2-3-7-18(17)23-10-4-5-11-23;/h8-9,12,17-18H,2-7,10-11,13H2,1H3;1H/t17-,18-;/m0./s1
|
| 化学名 |
2-(3,4-dichlorophenyl)-N-methyl-N-[(1S,2S)-2-pyrrolidin-1-ylcyclohexyl]acetamide;hydrochloride
|
| 别名 |
(-)-U-50488 HYDROCHLORIDE; 114528-79-9; (-)-trans-(1S,2S)-U-50488 hydrochloride; CHEMBL593781; (-)-trans-(1S,2S)-u-50488 Hydrochloride potent k opioid recep; 2-(3,4-dichlorophenyl)-N-methyl-N-[(1S,2S)-2-pyrrolidin-1-ylcyclohexyl]acetamide;hydrochloride; SR-01000075483; SR-01000597740;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O: 50 mg/mL (123.22 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4643 mL | 12.3216 mL | 24.6433 mL | |
| 5 mM | 0.4929 mL | 2.4643 mL | 4.9287 mL | |
| 10 mM | 0.2464 mL | 1.2322 mL | 2.4643 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。