| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
iron chelator; α-synuclein aggregation
|
|---|---|
| 体外研究 (In Vitro) |
PBT434 甲磺酸盐(0–20 µM;3 小时)可极大地抑制铁生成的 H2O2,并显着降低 Fe 介导的 α-突触核蛋白聚集速率 [1]。 PBT434 甲磺酸盐(0-100 µM;24 小时)不会对脑微血管内皮细胞产生细胞毒性影响 [2]。 PBT434 甲磺酸盐(20 µM;24 小时)可增加 hBMVEC 中总 TfR 和 Cp 蛋白水平的表达 [2]。
在这项研究中,研究人员确定铁螯合剂PBT434,目前正在开发用于治疗帕金森病和多系统萎缩,通过螯合细胞外Fe2+调节人脑微血管内皮细胞(hBMVEC)对铁的摄取。用PBT434治疗hBMVEC可增加转铁蛋白受体(TfR)和铜蓝蛋白(Cp)转录本的丰度。Western blot和ELISA分析也显示相应的蛋白增加。在细胞内,PBT434增加了可螯合的、不稳定的Fe2+的可检测水平;数据表明这些铁离子从铁蛋白中释放出来。此外,PBT434可能由于胞质亚铁铁(铁输出物的底物)转运蛋白的增加而增强铁的外排。PBT434在hBMVEC血脑屏障上快速双向平衡。这些结果表明,PBT434-铁复合物不是hBMVEC摄取的底物,因此支持PBT434螯合间质铁并抑制血脑屏障内皮细胞对铁的再摄取,以及抑制神经血管单元其他细胞对铁的摄取的模型。总的来说,这提出了一种新的和有前途的铁螯合治疗机制。[2] |
| 体内研究 (In Vivo) |
PBT434 甲磺酸盐(30 mg/kg;口服;每天一次,持续 21 天)在 L-DOPA 范式中表现出显着减少的旋转,在 MPTP 模型中大大减少 SNpc 神经元损失,在 6-OHDA 毒性模型中显着保留神经元数量。 [1]。
在体内,PBT434不会消耗正常啮齿动物的组织铁储备,但可以防止黑质致密部神经元(SNpc)的丢失,降低黑质α-突触核蛋白的积累,并在暴露于帕金森毒素6-OHDA和MPTP的小鼠以及PD的转基因动物模型(hA53T α-突触核蛋白)中恢复运动性能。这些改善与氧化损伤标志物的减少、铁转运蛋白(铁出口物)和DJ-1水平的增加有关。研究人员得出结论,针对组织中不以高亲和力复合物形式存在的病理性铁池而设计的化合物可以维持SNpc神经元的存活,并可能改善PD的疾病。 |
| 酶活实验 |
α-synuclein聚集试验[1]
每批合成的重组α突触核蛋白都进行了蛋白质测序和质谱分析以确保其纯度。冻干纯化的WT重组α突触核蛋白用Tris缓冲盐水(TBS) pH 7.4进行重组。混合的等分液在10万g下在4°旋转30分钟,以去除预先形成的聚集体/种子。收集含有单体形式的上清并用于测定。用BCA法测定蛋白质浓度,称取硝酸铁,溶解于TBS溶液中。PBT434 溶于100% DMSO中,然后用毫水稀释成原液。在每个试管上按等浓度依次加入TBS、Fe、Compound/Veh和α synuclein。α - synuclein、Fe和复合物的最终浓度为186.6 μM。 一旦所有的溶液都在管中,样品在电镀前被涡流2秒。样品在ThT (20 μM)存在下检测。在37°的Perkin-Elmer Enspire多模式平板阅读器上读取,每30分钟(1800秒)读取一次,每次读取之间以800转/分(1800秒)震动,直至42小时。在波长450发射和485 nm激发下随时间测量ThT荧光强度。RFU值归一化为TBS ThT空白井,并随时间绘制。滞后时间和最大相对荧光单位(RFU)作为化合物动力学分析的指标。 电位法[1] 肽的电位滴定在MettlerTitrando 907/Dosino 800滴定系统上进行,使用InLab 422组合玻璃- ag /AgCl电极,每天通过硝酸滴定校准。0.1 M NaOH(不含二氧化碳)作为滴定剂。样品体积为1.2-1.5 ml。样品通常含有0.8 mM PBT434 ,溶解在4 mM HNO3/96 mM KNO3中。研究了Fe (II)和Fe (III)络合物的形成,使用2.5 - 4倍过量的化合物超过金属离子,作为硝酸盐添加。所有实验均在25℃氩气条件下进行,pH范围为2.3 ~ 12.2。使用HYPERQUAD程序对收集的数据进行分析[1]。同时计算了3 ~ 5次滴定,分别用于质子化、Fe (II)和Fe (III)络合。 在Cary 50或Perkin Elmer分光光度计上记录25°C下的紫外可见光谱,光谱范围为230-800 nm。所有实验的光程均为1 cm。对单独含有PBT434 离子或与Fe (II)、Fe (III)、Cu (II)或Zn (II)离子的样品,在pH为2.0-12.0范围内用NaOH滴定,小心地手动添加极少量的浓碱溶液。对于Fe (III)和Fe (II),使用PBT434 浓度为0.1 mM,配体与金属的比例为4:1,以符合提供良好电位滴定的条件。对于Cu (II),采用<强>PBT434 浓度为0.1 mM,配体与金属的比例为1:1 ~ 4:1。对于Zn (II),为了避免沉淀,在0.04 mM PBT434 和0.02 mM Zn (II)的较低浓度下进行光谱滴定。铁(II)样品在氮气下制备,在Coy手套箱中,并转移到分光光度计。 |
| 细胞实验 |
细胞毒性测定[2]
细胞类型: hBMVEC 测试浓度: 1、10、20、50、100 µM 孵育持续时间:24小时 实验结果:证明对脑微血管内皮细胞没有细胞毒性作用。 蛋白质印迹分析[2] 细胞类型: hBMVEC 测试浓度: 20 μM 孵育时间: 24 h 实验结果: 总TfR、Cp蛋白表达水平增加。 MTT测定[2] 将hBMVEC在24孔板中培养至融合,然后在细胞培养基中以 浓度的PBT434 在37℃下处理24h。第二天,取出培养基,用含有MTT (0.5mg/ml)的RPMI+血清培养基在37℃下孵育2h,再用10% SDS/0.01N HCl在37℃下孵育16h,使MTT甲醛晶体溶解。溶解后,将溶液分三次转移到96孔板上,在平板阅读器上读取570nm处的吸光度。对空白值进行校正,并与未处理的对照组归一化。0.1% Triton X-100处理的细胞作为细胞死亡的阳性对照。 14C-PBT434积累和流出试验[2] 为了摄取14C-PBT434, hBMVEC单层膜在RPMI1640加血清生长培养基中加载20 μM 14C-PBT434 ,在37℃下加载3h。如前所述,反应用冰冷的淬火缓冲液淬火,并在裂解缓冲液中裂解。对裂解物进行14C计数(Beckman LS6500闪烁计数器),并归一化为BCA测定的蛋白质含量。 对于14C-PBT434 外排,hBMVEC单层膜上载20 μM的14C-PBT434 ,在RPMI加血清生长培养基中37℃孵育30min,然后用预温的RPMI加柠檬酸盐洗涤两次,再在RPMI加血清外排培养基中孵育2.5h。每隔30min,用冷冻缓冲液对细胞进行淬灭,裂解后进行如上处理。细胞相关14C计数与蛋白质含量归一化。 在14C-PBT434 轨迹试验中,hBMVEC生长在transwell刀片的顶室中,顶室(RPMI+血清)或基底室(RPMI-血清)中分别加载20 μM 14C-PBT434。在指定时间点分别从顶室和基室采集培养基样品,3h后用冷淬缓冲液淬灭细胞,裂解细胞,同上处理。为了粗略估计细胞内<强>PBT434 浓度,根据初始播种密度估计汇合时200K - 250K细胞,内皮细胞体积近似为10,000 μm3,从细胞中剩余的14C-PBT434 pmol计算出浓度。 |
| 动物实验 |
Animal/Disease Models: 12 weeks, 25 g, Male C57BL/6 J mice (6-OHDA intoxication model)[1]
Doses: 30 mg/kg Route of Administration: Po; daily for 21 days (commencing 3 days following induction of lesion) Experimental Results: Prevented neuronal loss following 6-OHDA, preserving up to 75% of the SNpc neurons remaining (both Nissl and tyrosine hydroxylase (TH) positive neurons) after the initial phase of cell death. Animal/Disease Models: 12 weeks, 25 g, Male C57BL/ 6 J mice (MPTP model)[1] Doses: 1, 3, 10, 30, 80 mg/kg Route of Administration: Po; daily for 21 days (commenced 24 h after induction of lesion) Experimental Results: Increased the proportion of SNpc cells rescued , increased there was a trend to improved turning behavior, Dramatically increased varicosity abundance, prevented the decline in levels of the presynaptic marker synaptophysin (SYNP) in a dose-dependent manner. 6-OHDA intoxication model[1] Mice anesthetized with 2.5–3% isoflurane were placed into a stereotaxic apparatus and 3.0 μg of 6-OHDA was injected into the right SNpc, as described before. Amphetamine induced (5 mg/kg) rotational behavior was measured three days after 6-OHDA lesion using an automated Rotacounter system. Robust rotational behavior has been observed as early as one-day post-lesion. Only mice that exhibited rotations at day 3 between 200 and 450 times per hour were included in the trial. Mice were then randomly assigned to the PBT434 treatment group or sham-vehicle (VEH) treatment group. The PBT434 treatment group was gavaged at 30 mg/kg/day, commencing 3 days following induction of lesion. Experimenters were blinded to the assignment of treatments for each of the groups. Mice were retested and then culled twenty-one days post 6-OHDA lesion. MPTP model[1] Mice were administered an acute dosing regimen of four injections of MPTP two hours apart. Each experimental trial contained MPTP lesioned animals that were randomly subdivided into a sham treated group (vehicle alone) and drug treatment group (30 mg/kg/day PBT434 , commencing 24 h after MPTP until culled at day 21). Experimenters were blinded to the assignment of treatments for each of the groups. In one group of animals the mice were treated with analog of PBT434 (PBT434-met 30 mg/kg/day) which does not have the ability to bind metals as a control. Cerebrospinal fluid collection from dogs[1] The collection of cerebrospinal fluid (CSF) was performed at the conclusion of a 28 day toxicology study in 10 month old Beagle dogs. PBT434 was administered by oral gavage once a day for 28 days at the following doses: vehicle control (0 mg/kg/day), 10 mg/kg/day, 30 mg/kg/day and 50 mg/kg/day. Each treatment arm included 3 male and 3 female dogs. The CSF was extracted at necropsy into collection tubes containing 10uL of butylated hydroxytoluene, frozen on dry ice and stored at −80 °C until analysis. Any samples showing signs of hemolysis were excluded due to the potential for contamination of α-synuclein from blood [52]. Cerebrospinal fluid collection from rats[1] Cannulas were inserted into the lateral cerebral ventricles of wild-type rats by stereotactic surgery. CSF sampling was performed using a rodent microdialysis bowl (BASi, n = 8). Baseline CSF was sampled after which the animals were gavaged with PBT434 at 30 mg/kg. CSF samples were collected at one and four hours after gavage with PBT434 . Samples were analysed by Western blot for the presence of α-synuclein as described. |
| 参考文献 |
|
| 其他信息 |
Genetic and experimental evidence strongly implicate α-synuclein in the etiology of Parkinson’s disease, recommending this protein as a plausible target for potential disease modifying therapies. As understanding of the role of iron in the pathological process in PD evolves, evidence is emerging that α-synuclein levels may be modulated by selective targeting of this ubiquitous biometal. PBT434 was developed to exploit this therapeutic niche and in addition to its potential utility in the clinic will be a valuable tool for studying the role of metals in modulating α-synuclein levels, the role of oxidative stress as an initiator and perpetuator of the nigral lesion and the involvement of other components of the neuronal iron trafficking apparatus. Treatments currently available for PD and the atypical Parkinsonian conditions at best provide limited symptomatic relief and do not alter disease progression. The beneficial effects of PBT434 on motor function, neuropathology and biochemical markers of disease state in three different animal models of PD suggest disease modifying potential.[1]
In summary, we provide in vitro evidence for the trafficking of PBT434 across the BBB and into the brain interstitium consistent with the results of early phase clinical trials. Additionally, we show that while PBT434 has moderate effects on the LIP and regulation of downstream iron-dependent protein expression, it does not significantly interrupt normal cell physiology, unlike high affinity iron chelators. In addition, PBT434 is able to bind and redistribute extracellular ionic Fe2+, limiting the downstream oxidative stress associated with this pro-oxidant and its role in cytotoxic protein aggregation. This novel mechanism of action provides a compelling case for the continued development of PBT434 as a therapeutic agent in neurodegenerative diseases correlated with metal accumulation.[2] |
| 分子式 |
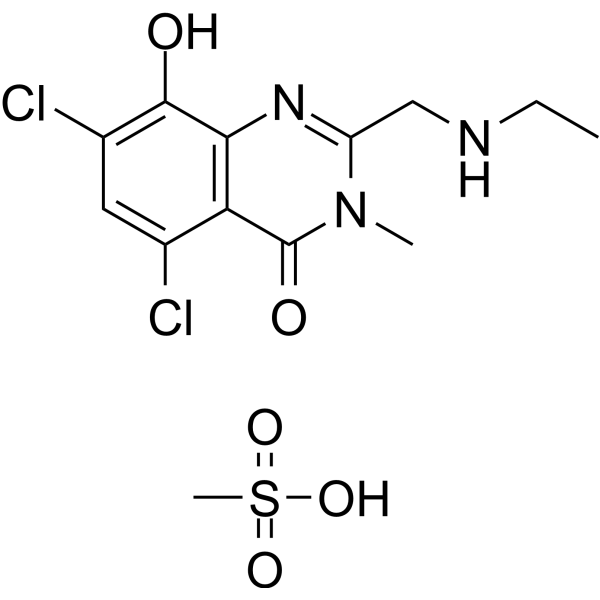
C13H17CL2N3O5S
|
|---|---|
| 分子量 |
398.262180089951
|
| 精确质量 |
397.026
|
| 元素分析 |
C, 39.21; H, 4.30; Cl, 17.80; N, 10.55; O, 20.09; S, 8.05
|
| CAS号 |
2387898-69-1
|
| 相关CAS号 |
1232840-87-7; 2387898-69-1 (mesylate); 1232841-78-9 (HBr)
|
| PubChem CID |
139593496
|
| 外观&性状 |
White to off-white solid powder
|
| tPSA |
128Ų
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
24
|
| 分子复杂度/Complexity |
484
|
| 定义原子立体中心数目 |
0
|
| SMILES |
S(O)(=O)(=O)C.O=C1N(C(CNCC)=NC2=C(C(Cl)=CC(Cl)=C12)O)C
|
| InChi Key |
UBTJWJNTOFSHON-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C12H13Cl2N3O2.CH4O3S/c1-3-15-5-8-16-10-9(12(19)17(8)2)6(13)4-7(14)11(10)18;1-5(2,3)4/h4,15,18H,3,5H2,1-2H3;1H3,(H,2,3,4)
|
| 化学名 |
5,7-dichloro-2-(ethylaminomethyl)-8-hydroxy-3-methylquinazolin-4-one;methanesulfonic acid
|
| 别名 |
PBT434 MESYLATE; PBT434 (methanesulfonate); 2387898-69-1; ATH434 MESYLATE; ATH-434 MESYLATE; ATH434; ATH-434; ATH 434; PBT-434 MESYLATE; 826P1VAG3U; EX-A8324;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5109 mL | 12.5546 mL | 25.1092 mL | |
| 5 mM | 0.5022 mL | 2.5109 mL | 5.0218 mL | |
| 10 mM | 0.2511 mL | 1.2555 mL | 2.5109 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。