| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| Other Sizes |
| 靶点 |
Histamine H1-receptor, Noradrenaline uptake (α1-and α2-adrenoceptors and serotonin 5HT1 and 5HT2-receptors)[1]
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Dosulepin is well absorbed from the intestines to reach the peak plasma concentration of 37.6ng/mL at 2.18 hours (Tmax) following oral administration of 25mg. The steady state concentrations are variable among individuals due to dynamic relationship between the drug dose and plasma concentration. Dosulepin is predominantly cleared via renal elimination, mainly in the form of metabolites. Renal excretion of dosulepin and its metabolites accounts for 50% - 60% of total elimination, and biliary/fecal excretion is about 15%-40%. The mean apparent Vd is approximately 45 L/kg after oral administration of 75mg dosulepin. It crosses the blood-brain barrier to mediate its antidepressant actions and also crosses the placental barriers, with low concentration of the drug excreted in breast milk. Oral clearance is approximately 1.36 L/kg hr following a single oral dose of 75mg dosulepin. Dothiepin and its metabolites have been detected in breast milk. The mean total daily infant exposure amounts to about 4.4% of the maternal dothiepin dosage. Dothiepin is readily absorbed in the gastrointestinal tract. The pharmacokinetics of dothiepin were evaluated in 9 depressed patients following a single oral dose of 75 mg. Blood and plasma concentrations of dothiepin and 2 major metabolites, northiaden and dothiepin S-oxide, were measured by gas chromatography/mass fragmentography. The mean (+/-SD) peak plasma concentrations of dothiepin were 49 +/- 27 ug/L at 3 +/- 1.2hr. Mean (+/-SD) estimates of other parameters were as follows: absorption half-life 1.1 +/- 1.1hr; distribution half-life 2.2 +/- 0.8 hr; elimination half-life 25 +/- 7hr; apparent volume of distribution 70 +/- 62 L/kg; and oral clearance 2.1 +/- 1.6 L/kg/hr. The mean (+/-SD) peak plasma concentration of dothiepin S-oxide was 125 +/- 43 ug/L at 3.5 +/- 1.3hr with an elimination half-life of 22 +/- 12 hr. The mean peak plasma concentration of northiaden was 6 +/- 3 ug/L at 4.5 +/- 1.1hr, with an elimination half-life of 31 +/- 12 hr. No significant differences were found in pharmacokinetic parameters compared with a previous study in 7 healthy volunteers. When data for the patients and healthy volunteers were combined (n = 16), pharmacokinetic parameters were not found to be affected by age. However, a significant difference was found between males and females for the elimination half-lives of dothiepin and northiaden, and for the apparent volume of distribution of dothiepin. The 24-hour blood/plasma concentrations of dothiepin and dothiepin S-oxide accurately predicted the steady-state concentrations obtained following 4 weeks' treatment with dothiepin 150 mg /at night/. Twenty-seven healthy men received three single oral doses of 50-, 100-, and 150-mg dothiepin hydrochloride capsules in a three-way randomized, crossover dose-proportionality study. Plasma concentration-time profiles of dothiepin (1) were described by both one- and two-compartment models with first-order absorption. The total intrinsic clearance of dothiepin decreased from 165.5 to 121.1 L/hr as the dose was increased from 50 to 150 mg, but there was no significant effect on the terminal half-life (approximately 20 hr). Plasma concentration-time profiles of the three major metabolites of dothiepin, the S-oxide derivative of dothiepin, N,N-dimethyl[b,e]thiepin-delta 11(6 H), gamma-propylamine 5-oxide (2), the demethyl derivative, N-methyldibenzo[b,e]thiepin-delta 11(6 H), gamma-propylamine (3) and the demethyl S-oxide derivative N-methyldibenzo[b,e]thiepin-delta 11(6 H), gamma-propylamine 5-oxide (4), were described by a one-compartment model with apparent first-order formation. The AUC infinity values of the S-oxide 2 and the demethyl S-oxide 4 increased proportionally with dose. The dose proportionality of the demethyl metabolite 3 may not be ascertained from the data in this study. The corresponding half-lives of the three metabolites, which are dose independent, were approximately 24, 28, and 40 hr, respectively. /Dothiepin Hydrochloride/ For more Absorption, Distribution and Excretion (Complete) data for DOTHIEPIN (6 total), please visit the HSDB record page. Metabolism / Metabolites Dosulepin undergoes extensive hepatic metabolism, to form main metabolites N-demethylated derivative northiaden (desmethyldosulepin or northiaden) and dosulepin S-oxide. Northiaden S-oxide is among 12 basic metabolites that are found in urine. The metabolic pathways of dosulepin is thought to involve N-demethylation, S-oxidation and glucuronic acid conjugation. Dothiepin-X-oxide is the major metabolite. Northiaden (desmethyl-dothiepin) is an N-demethylated derivative. Both metabolites have antidepressant activity. Twenty-seven healthy men received three single oral doses of 50-, 100-, and 150-mg dothiepin hydrochloride capsules in a three-way randomized, crossover dose-proportionality study. ... Plasma concentration-time profiles of the three major metabolites of dothiepin, the S-oxide derivative of dothiepin, N,N-dimethyl[b,e]thiepin-delta 11(6 H), gamma-propylamine 5-oxide (2), the demethyl derivative, N-methyldibenzo[b,e]thiepin-delta 11(6 H), gamma-propylamine (3) and the demethyl S-oxide derivative N-methyldibenzo[b,e]thiepin-delta 11(6 H), gamma-propylamine 5-oxide (4), were described by a one-compartment model with apparent first-order formation. The AUC infinity values of the S-oxide 2 and the demethyl S-oxide 4 increased proportionally with dose. The dose proportionality of the demethyl metabolite 3 may not be ascertained from the data in this study. The corresponding half-lives of the three metabolites, which are dose independent, were approximately 24, 28, and 40 hr, respectively. /Dothiepin Hydrochloride/ Only small amounts of unconjugated dothiepin (unchanged drug) and northiaden were excreted in urine over a 72 hr period. More than 10% of the dose was excreted as conjugated dothiepin and less than 0.8% of the dose as conjugated northiaden. Conjugated dothiepin was thus found to be an important metabolite of dothiepin. Conjugated dothiepin and northiaden were hydrolyzed with beta-glucuronidase, and their hydrolysis inhibited with 1,4 saccharolactone. Conjugated dothiepin and northiaden were found to be a quaternary ammonium-linked glucuronide and a tertiary N-glucuronide, respectively. ... Plasma and blood concentrations of northiaden and blood concentrations of dothiepin S-oxide, two metabolites of dothiepin, were measured. Dothiepin S-oxide was the major metabolic reaching a peak level of 81 (34-150) ug/l at 5 (4-6) hr. In comparison, northiaden reached a peak concentration of only 10 (3-21) ug/l at 5 (4-9) hr. The mean half-life of elimination of dothiepin S-oxide was 19 (13-35) hr while that for northiaden was 33 (22-60) hr. Biological Half-Life The elimination half life is approximately 20.4 hours following oral administration of 25mg dosulepin. The beta half-life (elimination) is about 20 hours. The mean half-life of absorption of 1.2 hours. The distribution half-life is 2.6 hours. Seven healthy volunteers received a single oral dose of 75 mg dothiepin. ... Mean estimates were as follows: absorption half life 1.2(0.07-3.0) hr, distribution half-life 2.6 (1.1-3.8) hr, elimination half-life 22 (14-40) hr ... . Plasma and blood concentrations of northiaden and blood concentrations of dothiepin S-oxide, two metabolites of dothiepin, were also measured. ... The mean half-life of elimination of dothiepin S-oxide was 19 (13-35) hr while that for northiaden was 33 (22-60) hr. |
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
Seizures or arrhythmias may be precipitated /SRP: by flumazenil/ in patients with a serious cyclic antidepressant overdose. /Flumazenil/ |
| 参考文献 | |
| 其他信息 |
Dothiepin is a dibenzothiepine. It has a role as an antidepressant and an anticoronaviral agent.
Dosulepin (INN, BAN) formerly known as dothiepin (USAN), is a tricyclic antidepressant with anxiolytic properties that is used in several European and South Asian countries, as well as Australia, South Africa, and New Zealand. It is not FDA-approved due to low therpeutic index and significant toxicity in overdose. Dosulepin inhibits the reuptake of biogenic amines, increasing available neurotransmitter levels at the synaptic cleft. The use of dosulepsin is only recommended in patients who are intolerant or unresponsive to alternative antidepressant therapies. Dosulepsin is a thio derivative of [DB00321] with a similar efficacy to that of [DB00321], and also exhibits anticholinergic, antihistamine and central sedative properties. Its hydrochloride form is a common active ingredient in different drug formulations. A tricyclic antidepressant with some tranquilizing action. Drug Indication Indicated in the treatment of symptoms of depressive illness, especially where an anti-anxiety effect is required. Mechanism of Action By binding to noradrenaline transporter (NAT) and serotonin transporter (SERT) in an equipotent manner and inhibiting the reuptake activity, dosulepin increases the free levels of noradrenaline and 5HT at the synaptic cleft. It is shown that the main metabolite northiaden is a more potent inhibitor of noradrenaline uptake than the parent drug. Dosulepin displays affinity towards α2-adrenoceptors and to a lesser extent, α1-adrenoceptors. Inhibition of presynaptic α2-adrenoceptors by dosulepin facilitates noradrenaline release and further potentiates the antidepressant effects. It also downregulates central β-adrenoceptors by causing a decline in the number of receptors and reduces noradrenaline-induced cyclic AMP formation. Dosulepin binds to 5HT1A and 5HT2A receptors in the cerebral cortex and hippocampus as an antagonist. 5HT1A receptors are autoreceptors that inhibit 5HT release and 5HT2A receptors are Gi/Go-coupled receptors that reduces dopamine release upon activation. Antagonism at 5HT2A receptors may also improve sleep patterns. Dosulepin also binds to muscarinic acetylcholine receptors and causes antimuscarinic side effects such as dry mouth. By acting as an antagonist at histamine type 1 (H1) receptors, dosulepin mediates a sedative effect. Main metabolites northiaden, dothiepin sulphoxide and northiaden sulphoxide may also bind to 5HT, α2 and H1 receptors, although with less affinity compared to the parent drug. Dothiepin is a tricyclic antidepressant that is structurally related to amitriptyline. It appears that the antidepressant activity of dothiepin is mediated through facilitation of noradrenergic neurotransmission by uptake inhibition and possibly also by enhancement of serotoninergic neurotransmission. The overall therapeutic efficacy of dothiepin is very similar to that of amitriptyline. Therapeutic Uses Dothiepin is a tricyclic antidepressant that is structurally related to amitriptyline. EXPTL Therapy: The effectiveness of dothiepin (a tricyclic anti-depressant) ... given orally at night was compared with placebo for 4 weeks in alleviating pain in 60 patients with classical or definite active rheumatoid arthritis. Patients were classified as either 'depressed' or 'not depressed'. The week before, during and 2 weeks after the study, 600 mg ibuprofen was given orally three times daily to all patients. Compared with placebo, dothiepin produced a significant reduction in daytime pain by the end of the treatment period. The Hamilton rating scale in 'depressed' patients was significantly improved in patients given dothiepin. The Cassano-Castrogiovanni self-evaluation rating scale in both 'depressed' and 'not depressed' patients showed a tendency (not significant) to be improved following dothiepin treatment compared with placebo. These results suggest that patients with rheumatoid arthritis may experience an increase in pain symptoms due to an alteration of mood. Therapy with tricyclic anti-depressants, such as dothiepin, therefore, may determine an improvement of pain indexes besides having an anti-depressant effect. Drug Warnings A case of inappropriate ADH syndrome associated with dothiepin therapy, initially given at a dose of 75 mg then increased to 150 mg/day, is reported in a 39-yr-old depressed man with pancytopenia and portal hypertension. The patient became drowsy, disoriented and confused, and had persistent hyponatremia and raised arginine-vasopressin levels. Dothiepin was stopped and the patient was treated with fluid restriction and subsequently, demeclocycline. Serum sodium and osmolality returned to normal in 4 days and arginine vasopressin concentrations returned to normal. To determine whether antidepressants are a risk factor for ischemic heart disease and to compare the risk for different subgroups of antidepressants and individual antidepressants. Case-control study. Nine general practices recruited from the Trent Focus Collaborative Research Network. 933 men and women with ischemic heart disease matched by age, sex, and practice to 5516 controls. Adjusted odds ratio for ischemic heart disease calculated by logistic regression. Odds ratios for ischemic heart disease were significantly raised for patients who had ever received a prescription for tricyclic antidepressants even after diabetes, hypertension, smoking, body mass index, and use of selective serotonin reuptake inhibitors had been adjusted for (1.56; 95% confidence interval 1.18 to 2.05). Patients who had ever taken dosulepin (dothiepin) had a significantly raised odds ratio for ischemic heart disease after adjustment for confounding factors and use of other antidepressants (1.67, 1.17 to 2.36). ... Increasing maximum doses of dosulepin were associated with increasing odds ratios for ischemic heart disease. Similarly, there was a significant positive trend associated with increasing numbers of prescriptions of dosulepin (adjusted odds ratio 1.52 for 1 prescription, 1.39 for 2-3, and 1.96 for >/=4, P<0.002). There is good evidence for an association between dosulepin and subsequent ischemic heart disease and for a dose-response relation. Pharmacodynamics Dosulepin is a tricyclic antidepressant that interacts with various receptors and transporters. It is a monoamine reuptake inhibitor with approximately equal potency for noradrenaline and 5-HT that increases the availability of these neurotransmitters at the central synapses. The metabolites of dosulepin are shown to inhibit 5HT uptake by the human blood platelet. |
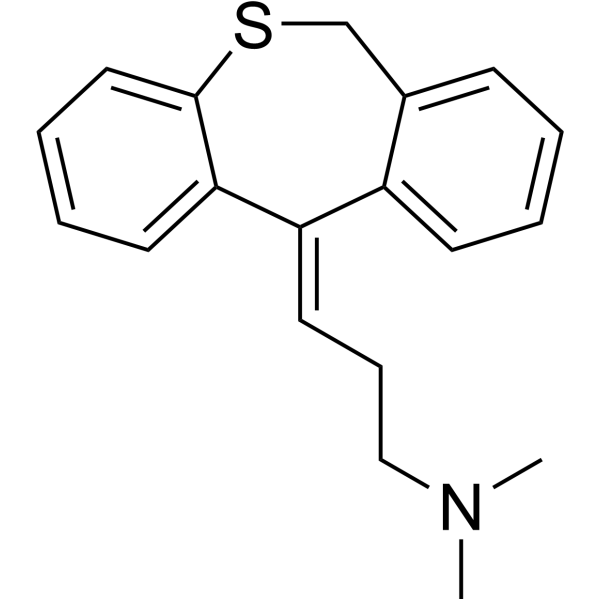
| 分子式 |
C19H21NS
|
|---|---|
| 分子量 |
295.44
|
| 精确质量 |
295.139
|
| CAS号 |
113-53-1
|
| 相关CAS号 |
Dothiepin-d3;136765-31-6;Dothiepin-d6 hydrochloride;1276545-35-7
|
| PubChem CID |
5282426
|
| 外观&性状 |
Off-white to yellow solid powder
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
430.9±44.0 °C at 760 mmHg
|
| 熔点 |
55-57ºC
|
| 闪点 |
214.4±28.4 °C
|
| 蒸汽压 |
0.0±1.0 mmHg at 25°C
|
| 折射率 |
1.661
|
| LogP |
4.34
|
| tPSA |
28.54
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
21
|
| 分子复杂度/Complexity |
363
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CN(C)CC/C=C\1/C2=CC=CC=C2CSC3=CC=CC=C31
|
| InChi Key |
PHTUQLWOUWZIMZ-BOPFTXTBSA-N
|
| InChi Code |
InChI=1S/C19H21NS/c1-20(2)13-7-11-17-16-9-4-3-8-15(16)14-21-19-12-6-5-10-18(17)19/h3-6,8-12H,7,13-14H2,1-2H3/b17-11-
|
| 化学名 |
(3Z)-3-(6H-benzo[c][1]benzothiepin-11-ylidene)-N,N-dimethylpropan-1-amine
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.3848 mL | 16.9239 mL | 33.8478 mL | |
| 5 mM | 0.6770 mL | 3.3848 mL | 6.7696 mL | |
| 10 mM | 0.3385 mL | 1.6924 mL | 3.3848 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。