| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
α-adrenergic receptor[1]; (-)-Scopolamine (Atroscine) acts on α1 - adrenergic receptor and muscarinic cholinergic receptor, with Ki values of 33 μM and 7.25 nM respectively [1]
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The pharmacokinetics of scopolamine differ substantially between different dosage routes. Oral administration of 0.5 mg scopolamine in healthy volunteers produced a Cmax of 0.54 ± 0.1 ng/mL, a tmax of 23.5 ± 8.2 min, and an AUC of 50.8 ± 1.76 ng\*min/mL; the absolute bioavailability is low at 13 ± 1%, presumably because of first-pass metabolism. By comparison, IV infusion of 0.5 mg scopolamine over 15 minutes resulted in a Cmax of 5.00 ± 0.43 ng/mL, a tmax of 5.0 min, and an AUC of 369.4 ± 2.2 ng\*min/mL. Other dose forms have also been tested. Subcutaneous administration of 0.4 mg scopolamine resulted in a Cmax of 3.27 ng/mL, a tmax of 14.6 min, and an AUC of 158.2 ng\*min/mL. Intramuscular administration of 0.5 scopolamine resulted in a Cmax of 0.96 ± 0.17 ng/mL, a tmax of 18.5 ± 4.7 min, and an AUC of 81.3 ± 11.2 ng\*min/mL. Absorption following intranasal administration was found to be rapid, whereby 0.4 mg of scopolamine resulted in a Cmax of 1.68 ± 0.23 ng/mL, a tmax of 2.2 ± 3 min, and an AUC of 167 ± 20 ng\*min/mL; intranasal scopolamine also had a higher bioavailability than that of oral scopolamine at 83 ± 10%. Due to dose-dependent adverse effects, the transdermal patch was developed to obtain therapeutic plasma concentrations over a longer period of time. Following patch application, scopolamine becomes detectable within four hours and reaches a peak concentration (tmax) within 24 hours. The average plasma concentration is 87 pg/mL, and the total levels of free and conjugated scopolamine reach 354 pg/mL. Following oral administration, approximately 2.6% of unchanged scopolamine is recovered in urine. Compared to this, using the transdermal patch system, less than 10% of the total dose, both as unchanged scopolamine and metabolites, is recovered in urine over 108 hours. Less than 5% of the total dose is recovered unchanged. The volume of distribution of scopolamine is not well characterized. IV infusion of 0.5 mg scopolamine over 15 minutes resulted in a volume of distribution of 141.3 ± 1.6 L. IV infusion of 0.5 mg scopolamine resulted in a clearance of 81.2 ± 1.55 L/h, while subcutaneous administration resulted in a lower clearance of 0.14-0.17 L/h. Scopolamine hydrobromide is rapidly absorbed following IM or subcutaneous injection. The drug is well absorbed from the GI tract, principally from the upper small intestine. Scopolamine also is well absorbed percutaneously. Following topical application behind the ear of a transdermal system, scopolamine is detected in plasma within 4 hours, with peak concentrations occurring within an average of 24 hours. In one study in healthy individuals, mean free and total (free plus conjugated) plasma scopolamine concentrations of 87 and 354 pg/mL, respectively, have been reported within 24 hours following topical application of a single transdermal scopolamine system that delivered approximately 1 mg/72 hours. /Scopolamine hydrobromide/ Following oral administration of a 0.906-mg dose of scopolamine in one individual, a peak concentration of about 2 ng/mL was reached within 1 hour. Although the commercially available transdermal system contains 1.5 mg of scopolamine, the membrane-controlled diffusion system is designed to deliver approximately 1 mg of the drug to systemic circulation at an approximately constant rate over a 72-hour period. An initial priming dose of 0.14 mg of scopolamine is released from the adhesive layer of the system at a controlled, asymptotically declining rate over 6 hours; then, the remainder of the dose is released at an approximate rate of 5 ug/hour for the remaining 66-hour functional lifetime of the system. The manufacturer states that the initial priming dose saturates binding sites on the skin and rapidly brings the plasma concentration to steady-state. In a crossover study comparing urinary excretion rates of scopolamine during multiple 12-hour collection intervals in healthy individuals, there was no difference between the rates of excretion of drug during steady-state (24-72 hours) for constant-rate IV infusion (3.7-6 mcg/hour) and transdermal administration. The transdermal system appeared to deliver the drug to systemic circulation at the same rate as the constant-rate IV infusion; however, relatively long collection intervals (12 hours) make it difficult to interpret the data precisely. During the 12- to 24-hour period of administration and after 72 hours, the rate of excretion of scopolamine was higher with the transdermal system than with the constant-rate IV infusion. The distribution of scopolamine has not been fully characterized. The drug appears to be reversibly bound to plasma proteins. Scopolamine apparently crosses the blood-brain barrier since the drug causes CNS effects. The drug also reportedly crosses the placenta and is distributed into milk.. Although the metabolic and excretory fate of scopolamine has not been fully determined, the drug is thought to be almost completely metabolized (principally by conjugation) in the liver and excreted in urine. Following oral administration of a single dose of scopolamine in one study, only small amounts of the dose (about 4-5%) were excreted unchanged in urine within 50 hours; urinary clearance of unchanged drug was about 120 mL/minute. In another study, 3.4% or less than 1% of a single dose was excreted unchanged in urine within 72 hours following subcutaneous injection or oral administration of the drug, respectively. Following application of a single transdermal scopolamine system that delivered approximately 1 mg/72 hours in healthy individuals, the urinary excretion rate of free and total (free plus conjugated) scopolamine was about 0.7 and 3.8 ug/hour, respectively. Following removal of the transdermal system of scopolamine, depletion of scopolamine bound to skin receptors at the site of the application of the transdermal system results in a log-linear decrease in plasma scopolamine concentrations. Less than 10% of the total dose is excreted in urine as unchanged drug and its metabolites over 108 hours. Metabolism / Metabolites Little is known about the metabolism of scopolamine in humans, although many metabolites have been detected in animal studies. In general, scopolamine is primarily metabolized in the liver, and the primary metabolites are various glucuronide and sulphide conjugates. Although the enzymes responsible for scopolamine metabolism are unknown, _in vitro_ studies have demonstrated oxidative demethylation linked to CYP3A subfamily activity, and scopolamine pharmacokinetics were significantly altered by coadministration with grapefruit juice, suggesting that CYP3A4 is responsible for at least some of the oxidative demethylation. Although the metabolic and excretory fate of scopolamine has not been fully determined, the drug is thought to be almost completely metabolized (principally by conjugation) in the liver and excreted in urine. Route of Elimination: Less than 10% of the total dose is excreted in the urine as parent and metabolites over 108 hours. Half Life: 4.5 hours Biological Half-Life The half-life of scopolamine differs depending on the route. Intravenous, oral, and intramuscular administration have similar half-lives of 68.7 ± 1.0, 63.7 ± 1.3, and 69.1 ±8/0 min, respectively. The half-life is greater with subcutaneous administration at 213 min. Following removal of the transdermal patch system, scopolamine plasma concentrations decrease in a log-linear fashion with a half-life of 9.5 hours. Following application of a single transdermal scopolamine system that delivered approximately 1 mg/72 hours, the average elimination half-life of the drug was 9.5 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Scopolamine acts by interfering with the transmission of nerve impulses by acetylcholine in the parasympathetic nervous system (specifically the vomiting center). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of scopolamine during breastfeeding. Use during labor appears to have a detrimental effect on newborn infants' nursing behavior. Long-term use of scopolamine might reduce milk production or milk letdown, but a single systemic or ophthalmic dose is not likely to interfere with breastfeeding. During long-term use, observe for signs of decreased lactation (e.g., insatiety, poor weight gain). To substantially diminish the amount of drug that reaches the breastmilk after using eye drops, place pressure over the tear duct by the corner of the eye for 1 minute or more, then remove the excess solution with an absorbent tissue. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Anticholinergics can inhibit lactation in animals, apparently by inhibiting growth hormone and oxytocin secretion. Anticholinergic drugs can also reduce serum prolactin in nonnursing women. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. A retrospective case-control study conducted in two hospitals in central Iran compared breastfeeding behaviors in the first 2 hours postdelivery by infants of 4 groups of primiparous women with healthy, full-term singleton births who had vaginal deliveries. The groups were those who received no medications during labor, those who received oxytocin plus scopolamine, those who received oxytocin plus meperidine, and those who received oxytocin, scopolamine and meperidine. The infants in the no medication group performed better than those in all other groups, and the oxytocin plus scopolamine group performed better than the groups that had received meperidine. Protein Binding Scopolamine may reversibly bind plasma proteins in humans. In rats, scopolamine exhibits relatively low plasma protein binding of 30 ± 10%. Interactions Scopolamine should be used with care in patients taking other drugs that are capable of causing CNS effects such as sedatives, tranquilizers, or alcohol. Special attention should be paid to potential interactions with drugs having anticholinergic properties; e.g., other belladonna alkaloids, antihistamines (including meclizine), tricyclic antidepressants, and muscle relaxants. The absorption of oral medications may be decreased during the concurrent use of scopolamine because of decreased gastric motility and delayed gastric emptying. Concomitant administration of antimuscarinics and corticosteroids may result in increased intraocular pressure. /Antimuscarinics/Antispasmodics/ Antacids may decrease the extent of absorption of some oral antimuscarinics when these drugs are administered simultaneously. Therefore, oral antimuscarinics should be administered at least 1 hour before antacids. Antimuscarinics may be administered before meals to prolong the effects of postprandial antacid therapy. However, controlled studies have failed to demonstrate a substantial difference in gastric pH when combined antimuscarinic and antacid therapy was compared with antacid therapy alone. /Antimuscarinics/Antispasmodics/ For more Interactions (Complete) data for SCOPOLAMINE (8 total), please visit the HSDB record page. |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Adjuvants, Anesthesia; Antiemetics; Muscarinic Antagonists; Mydriatics; Parasympatholytics Although transdermal scopolamine has been shown to decrease basal acid output and inhibit betazole-, pentagastrin-, and peptone-stimulated gastric acid secretion in healthy individuals, it has not been determined whether transdermal scopolamine is effective in the adjunctive treatment of peptic ulcer disease. /Use is not currently included in the labeling approved by the US FDA/ Transdermal scopolamine has shown minimal antiemetic activity against chemotherapy-induced vomiting. /Use is not currently included in the labeling approved by the US FDA/ Scopolamine hydrobromide is used as a mydriatic and cycloplegic, especially when the patient is sensitive to atropine or when less prolonged cycloplegia is required. The effects of the drug appear more rapidly and have a shorter duration of action than those of atropine. Scopolamine hydrobromide is also used in the management of acute inflammatory conditions (i.e., iridocyclitis) of the iris and uveal tract. /Scopolamine hydrobromide/ For more Therapeutic Uses (Complete) data for SCOPOLAMINE (10 total), please visit the HSDB record page. Drug Warnings The use of scopolamine to produce tranquilization and amnesia in a variety of circumstances, including labor, is declining and of questionable value. Given alone in the presence of pain or severe anxiety, scopolamine may induce outbursts of uncontrolled behavior. Scopolamine in therapeutic doses normally causes CNS depression manifested as drowsiness, amnesia, fatigue, and dreamless sleep, with a reduction in rapid eye movement (REM) sleep. It also causes euphoria and is therefore subject to some abuse. The depressant and amnesic effects formerly were sought when scopolamine was used as an adjunct to anesthetic agents or for preanesthetic medication. However, in the presence of severe pain, the same doses of scopolamine can occasionally cause excitement, restlessness, hallucinations, or delirium. These excitatory effects resemble those of toxic doses of atropine. Scopolamine-induced inhibition of salivation occurs within 30 minutes or within 30 minutes to 1 hour and peaks within 1 or 1-2 hours after IM or oral administration, respectively; inhibition of salivation persists for up to 4-6 hours. Following IV administration of a 0.6-mg dose in one study, amnesia occurred within 10 minutes, peaked between 50-80 minutes, and persisted for at least 120 minutes after administration. Following IM administration of a 0.2-mg dose of scopolamine in one study, antiemetic effect occurred within 15-30 minutes and persisted for about 4 hours. Following IM administration of a 0.1- or 0.2-mg dose in another study, mydriasis persisted for up to 8 hours. The transdermal system is designed to provide an antiemetic effect with an onset of about 4 hours and with a duration of up to 72 hours after application. Small doses of ... scopolamine inhibit the activity of sweat glands innervated by sympathetic cholinergic fibers, and the skin becomes hot and dry. Sweating may be depressed enough to raise the body temperature, but only notably so after large doses or at high environmental temperatures. For more Drug Warnings (Complete) data for SCOPOLAMINE (21 total), please visit the HSDB record page. Pharmacodynamics Scopolamine is an anticholinergic belladonna alkaloid that, through competitive inhibition of muscarinic receptors, affects parasympathetic nervous system function and acts on smooth muscles that respond to acetylcholine but lack cholinergic innervation. Formulated as a patch, scopolamine is released continuously over three days and remains detectable in urine over a period of 108 hours. Scopolamine is contraindicated in angle-closure glaucoma and should be used with caution in patients with open-angle glaucoma due to scopolamine's ability to increase intraocular pressure. Also, scopolamine exhibits several neuropsychiatric effects: exacerbated psychosis, seizures, seizure-like, and other psychiatric reactions, and cognitive impairment; scopolamine may impair the ability of patients to operate machinery or motor vehicles, play underwater sports, or perform any other potentially hazardous activity. Women with severe preeclampsia should avoid scopolamine. Patients with gastrointestinal or urinary disorders should be monitored frequently for impairments, and scopolamine should be discontinued if these develop. Scopolamine can cause blurred vision if applied directly to the eye, and the transdermal patch should be removed before an MRI procedure to avoid skin burns. Due to its gastrointestinal effects, scopolamine can interfere with gastric secretion testing and should be discontinued at least 10 days before performing the test. Finally, scopolamine may induce dependence and resulting withdrawal symptoms, such as nausea, dizziness, vomiting, gastrointestinal disturbances, sweating, headaches, bradycardia, hypotension, and various neuropsychiatric manifestations following treatment discontinuation; severe symptoms may require medical attention. |
| 分子式 |
C17H21NO4
|
|---|---|
| 分子量 |
303.35
|
| 精确质量 |
303.147
|
| CAS号 |
138-12-5
|
| PubChem CID |
5184
|
| 外观&性状 |
Viscous liquid
|
| 密度 |
0.827 g/mL at 25 °C(lit.)
|
| 沸点 |
196 °C(lit.)
|
| 熔点 |
−15 °C(lit.)
|
| 闪点 |
178 °F
|
| 蒸汽压 |
0mmHg at 25°C
|
| 折射率 |
n20/D 1.429(lit.)
|
| LogP |
0.856
|
| tPSA |
62.3
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
22
|
| 分子复杂度/Complexity |
418
|
| 定义原子立体中心数目 |
0
|
| SMILES |
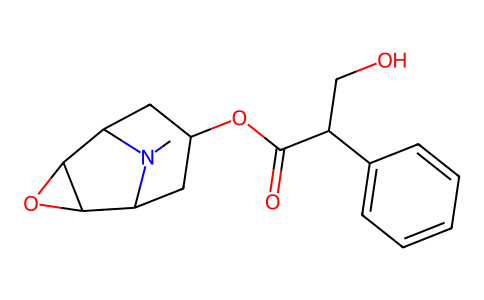
OCC(C1C=CC=CC=1)C(OC1CC2N(C)C(C3C2O3)C1)=O
|
| InChi Key |
STECJAGHUSJQJN-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C17H21NO4/c1-18-13-7-11(8-14(18)16-15(13)22-16)21-17(20)12(9-19)10-5-3-2-4-6-10/h2-6,11-16,19H,7-9H2,1H3
|
| 化学名 |
(9-methyl-3-oxa-9-azatricyclo[3.3.1.02,4]nonan-7-yl) 3-hydroxy-2-phenylpropanoate
|
| 别名 |
(-)-Scopolamine; scopalamine; scopolamine; 138-12-5; Atroscine; [(1S,2S,4R,5R)-9-methyl-3-oxa-9-azatricyclo[3.3.1.02,4]nonan-7-yl] 3-hydroxy-2-phenylpropanoate; 51-34-3; Benzeneacetic acid, alpha-(hydroxymethyl)-, 9-methyl-3-oxa-9-azatricyclo(3.3.1.0(2,4))non-7-yl ester, (1alpha,2beta,4beta,5alpha,7beta)-(+-)-;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.2965 mL | 16.4826 mL | 32.9652 mL | |
| 5 mM | 0.6593 mL | 3.2965 mL | 6.5930 mL | |
| 10 mM | 0.3297 mL | 1.6483 mL | 3.2965 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。