| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
|
| 靶点 |
Endogenous Metabolite
|
|---|---|
| 体外研究 (In Vitro) |
内源代谢物是京都基因和基因组百科全书确定为人类基因组中编码的约 1900 种代谢酶的产物或底物的代谢物。正如大量文献所证明的那样,许多这些代谢物已被证明具有有害影响[1]。
|
| 参考文献 |
[1]. Endogenous toxic metabolites and implications in cancer therapy. Oncogene. 2020 Aug;39(35):5709-5720.
[2]. Capillary electrophoresis-mass spectrometry-based metabolome analysis of serum and saliva from neurodegenerative dementia patients. Electrophoresis. 2013 Oct;34(19):2865-72. [3]. Intracellular flux analysis applied to the effect of dissolved oxygen on hybridomas. Appl Microbiol Biotechnol. 1995 Dec;44(1-2):27-36. |
| 其他信息 |
Isocitric acid is a tricarboxylic acid that is propan-1-ol with a hydrogen at each of the 3 carbon positions replaced by a carboxy group. It has a role as a fundamental metabolite. It is a tricarboxylic acid and a secondary alcohol. It is a conjugate acid of an isocitrate(1-).
Isocitric acid is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). Isocitric acid has been reported in Populus tremula, Vaccinium macrocarpon, and other organisms with data available. Isocitric acid is a metabolite found in or produced by Saccharomyces cerevisiae. It is well recognized that many metabolic enzymes play essential roles in cancer cells in producing building blocks such as nucleotides, which are required in greater amounts due to their increased proliferation. On the other hand, the significance of enzymes in preventing the accumulation of their substrates is less recognized. Here, we outline the evidence and underlying mechanisms for how many metabolites normally produced in cells are highly toxic, such as metabolites containing reactive groups (e.g., methylglyoxal, 4-hydroxynonenal, and glutaconyl-CoA), or metabolites that act as competitive analogs against other metabolites (e.g., deoxyuridine triphosphate and l-2-hydroxyglutarate). Thus, if a metabolic pathway contains a toxic intermediate, then we may be able to induce accumulation and poison a cancer cell by targeting the downstream enzyme. Furthermore, this poisoning may be cancer cell selective if this pathway is overactive in a cancer cell relative to a nontransformed cell. We describe this concept as illustrated in selenocysteine metabolism and other pathways and discuss future directions in exploiting toxic metabolites to kill cancer cells. [1] Despite increasing global prevalence, the precise pathogenesis and terms for objective diagnosis of neurodegenerative dementias remain controversial, and comprehensive understanding of the disease remains lacking. Here, we conducted metabolomic analysis of serum and saliva obtained from patients with neurodegenerative dementias (n = 10), including Alzheimer's disease, frontotemporal lobe dementia, and Lewy body disease, as well as from age-matched healthy controls (n = 9). Using CE-TOF-MS, six metabolites in serum (β-alanine, creatinine, hydroxyproline, glutamine, iso-citrate, and cytidine) and two in saliva (arginine and tyrosine) were significantly different between dementias and controls. Using multivariate analysis, serum was confirmed as a more efficient biological fluid for diagnosis compared to saliva; additionally, 45 metabolites in total were identified as candidate markers that could discriminate at least one pair of diagnostic groups from the healthy control group. These metabolites possibly provide an objective method for diagnosing dementia-type by multiphase screening. Moreover, diagnostic-type-dependent differences were observed in several tricarboxylic acid cycle compounds detected in serum, indicating that some pathways in glucose metabolism may be altered in dementia patients. This pilot study revealed novel alterations in metabolomic profiles between various neurodegenerative dementias, which would contribute to etiological investigations. [2] Quantitative estimates of intracellular fluxes and measurements of intracellular concentrations were used to evaluate the effect of dissolved oxygen (DO) concentration on CRL 1606 hybridoma cells in batch culture. The estimates of intracellular fluxes were generated by combining material balances with measurements of extracellular metabolite rates of change. Experiments were performed at DO levels of 60% and 1% air saturation, as well as under oxygen-limited conditions. Cell extracts were analyzed to evaluate the effect of DO on the intracellular concentrations of the glutamate dehydrogenase reactants, as well as the redox state of the pyridine nucleotides in the cytosol and mitochondria. The relationship between cell density and pyridine nucleotide redox state was also investigated. Dissolved oxygen concentration had a significant effect on nitrogen metabolism and the flux through glutamate dehydrogenase was found to reverse at low DO, favoring glutamate formation. The NAD in the cytosol and mitochondria was more reduced under low DO conditions while the cytosolic NAD was more oxidized at low DO. Cytosolic NAD was reduced at higher cell densities while the redox states of cytosolic NADP and mitochondrial NAD did not exhibit significant variation with cell density. These results point to the fundamental role of the intracellular oxidation/reduction state in cell physiology and the possibility of controlling physiological processes through modulation of the dissolved oxygen level or the oxidation/reduction potential of the culture. [3] |
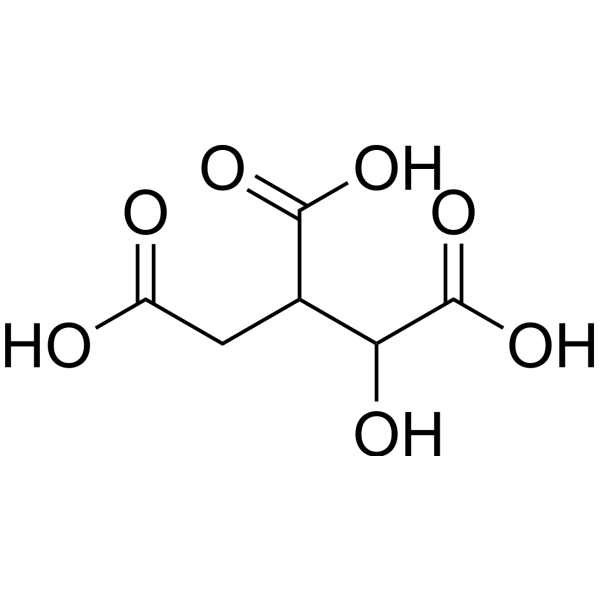
| 分子式 |
C6H8O7
|
|---|---|
| 分子量 |
192.12
|
| 精确质量 |
192.027
|
| CAS号 |
320-77-4
|
| 相关CAS号 |
DL-Isocitric acid trisodium salt;1637-73-6
|
| PubChem CID |
1198
|
| 外观&性状 |
Typically exists as White to off-white solid at room temperature
|
| 密度 |
1.751g/cm3
|
| 沸点 |
329.6ºC at 760 mmHg
|
| 熔点 |
162 - 165 °C
|
| 闪点 |
167.4ºC
|
| 折射率 |
1.569
|
| LogP |
-1.8
|
| tPSA |
132.13
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
13
|
| 分子复杂度/Complexity |
233
|
| 定义原子立体中心数目 |
0
|
| SMILES |
OC(CC(C(C(=O)O)O)C(=O)O)=O
|
| InChi Key |
ODBLHEXUDAPZAU-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C6H8O7/c7-3(8)1-2(5(10)11)4(9)6(12)13/h2,4,9H,1H2,(H,7,8)(H,10,11)(H,12,13)
|
| 化学名 |
1-hydroxypropane-1,2,3-tricarboxylic acid
|
| 别名 |
isocitric acid; 320-77-4; isocitrate; 1-Hydroxypropane-1,2,3-tricarboxylic acid; 3-Carboxy-2,3-dideoxy-1-hydroxypropan-1,2,3-tricarboxylic acid; DL-Isocitric acid; 3-carboxy-2,3-dideoxypentaric acid; 1-Hydroxytricarballylic acid;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.2051 mL | 26.0254 mL | 52.0508 mL | |
| 5 mM | 1.0410 mL | 5.2051 mL | 10.4102 mL | |
| 10 mM | 0.5205 mL | 2.6025 mL | 5.2051 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。