| 规格 | 价格 | ||
|---|---|---|---|
| 500mg | |||
| 1g | |||
| Other Sizes |
| 靶点 |
Caspase-1; IL-1β
|
|---|---|
| 体外研究 (In Vitro) |
Ac-YVAD-CHO 乙酸酯抑制人和小鼠 IL-1β,IC50 值分别为 2.5 和 0.7 μM [1]。 Ac-YVAD-CHO (0.01-100 μM) 乙酸酯可以降低 LPS 处理的血浆和腹膜液中 IL-1β 的升高 [1]。 Ac-YVAD-CHO (15.6 μM) 醋酸盐可减少 NO 介导的胸腺细胞凋亡 [3]。在用 SNAP 处理的胸腺细胞中,Ac-YVAD-CHO(15.6 μM,12 小时)醋酸盐可防止 NO 诱导的 PARP 裂解 [3]。
泛胱天蛋白酶抑制剂ZVAD-fmk和胱天蛋白酶-1抑制剂Ac-YVAD-CHO均以剂量依赖的方式抑制NO诱导的胸腺细胞凋亡,而胱天蛋白酶-3抑制剂Ac-DEVD-CHO即使在高达500微M的浓度下也几乎没有效果。在用NO供体S-亚硝基-N-乙酰青霉胺(SNAP)处理后,ZVAD-fmk和Ac-YVAD-CHO在添加12小时时能够抑制细胞凋亡,而不是16小时。Caspase-1活性在4小时和8小时上调,24小时恢复到基线水平;未检测到caspase-3活性。SNAP处理的胸腺细胞胞浆组分切割胱天蛋白酶激活的脱氧核糖核酸酶抑制剂。Ac-YVAD-cho完全阻断了这种切割,但Ac-DEVD-cho或DEVD-fmk没有阻断。SNAP处理后8小时和12小时,胸腺细胞中的聚ADP核糖聚合酶(PARP)也被切割;向培养物中添加Ac-YVAD-cho可阻断PARP切割。此外,SNAP诱导野生型小鼠44%的胸腺细胞凋亡;caspase-1敲除小鼠的胸腺细胞对NO诱导的凋亡更具抵抗力。这些数据表明,NO通过半胱天冬酶-1依赖而非半胱天冬蛋白酶-3依赖的途径诱导胸腺细胞凋亡。单独使用Caspase-1可以切割Caspase激活的脱氧核糖核酸酶抑制剂,导致DNA断裂,从而为NO诱导的胸腺细胞凋亡提供了一条新途径[3]。 |
| 体内研究 (In Vivo) |
Ac-YVAD-CHO(30 mg/kg;腹膜内;6 小时) 在对痤疮丙酸杆菌敏感的小鼠血液中,醋酸盐可降低 IL-1β 的水平 [1]。纹状体内输注 Ac-YVAD-CHO (2–8 μg) 喹啉酸 (QA) 诱导的大鼠纹状体细胞凋亡可被乙酸盐减弱 [2]。 Ac-YVAD-CHO(腹膜内注射,10 和 50 mg/kg,持续一小时)。乙酸盐迅速从循环中去除,并在注射后 30 至 60 分钟急剧下降至 1 至 0.2 μM [2]。
白细胞介素-1β转化酶(ICE)的一种强效、可逆的四肽抑制剂L-709049已被证明可以抑制成熟IL-1β的体外产生。我们现在报告说,这种抑制剂还有效地抑制了内毒素休克小鼠模型中成熟IL-1β的产生。腹腔注射L-709049以剂量相关方式降低了用LPS治疗的小鼠血浆和腹膜液中IL-1β的升高(ED50=2+/-0.9mg/kg)。LPS诱导的这些小鼠IL-1α和IL-6的升高不受影响,表明抑制剂特异性影响IL-1β的产生。血浆和腹腔液的免疫印迹分析表明,L-709049抑制了体内成熟IL-1β生成的形成。当小鼠血液在体外与LPS一起孵育时,IL-1β释放到血浆中。该测定用于测定小鼠服用ICE抑制剂后血液中ICE抑制剂的体外活性。小鼠腹腔注射10mg/kg L-709049 15分钟后获得的血液产生的IL-1β比对照血液少80%,注射该抑制剂30分钟后血液中IL-1β的产生恢复到对照水平。此外,从这些动物获得的血浆防止ICE切割合成底物的能力在ip施用50mg/kg抑制剂后1小时内消失。[1] 用Ac-YVAD-CHO预处理可抑制QA诱导的核小体间DNA断裂。Ac-YVAD-CHO抑制QA诱导的胱天蛋白酶-1活性和p53蛋白水平的增加,但对QA诱导的IκB-α降解、NF-κB或AP-1活化没有影响。 结论: Caspase-1参与QA诱导的p53上调,但不参与IκB-α的降解。抑制胱天蛋白酶-1可减弱QA诱导的大鼠纹状体细胞凋亡[2]。 |
| 酶活实验 |
在无细胞重建系统中诱导细胞凋亡[3]
该程序如前所述进行。反应混合物含有40μl S-100组分(约5mg/ml);10μl核溶液(约1×106个核);以及终浓度为80μl缓冲液F(10 mM HEPES(pH 7.4)、40 mM b-甘油磷酸、50 mM NaCl、2 mM MgCl2、4 mM EGTA、2 mM ATP、10 mM磷酸肌酸酐、50μg/ml肌酸酐激酶和0.2 mg/ml BSA)中的400 ng预处理的rh-天冬氨酸酶-1或rh-天冬酶-3、15.6μMAc-YVAD-CHO或15.6μM Ac-DEVD-CHO。混合物在37°C下孵育140分钟,偶尔混合。然后将反应溶液与500μl缓冲液G(50 mM Tris-HCl(pH 7.4)、1 mM EDTA、1%SDS和0.2 mg/ml蛋白酶K)混合,并在37°C下孵育1小时。用苯酚/氯仿提取溶液。如前所述进行DNA分离和电泳。 |
| 细胞实验 |
蛋白质印迹分析[3]
细胞类型: SNAP 处理的胸腺细胞 测试浓度: 15.6 μM 孵育时间: > 12 小时 实验结果:PARP 裂解减少。 |
| 动物实验 |
Animal/Disease Models: P. acnes-sensitized mice[1]
Doses: 50 mg/kg Route of Administration: Ip Experimental Results: Suppressed IL-1β levels in blood. Animal/Disease Models: Quinolinic acid-treated Rats[2] Doses: 2-8 μg Route of Administration: Intrastriatal infusion. Experimental Results: Attenuated Quinolinic acid (QA)-induced increases in p53 and apoptosis in rat striatum. Inhibited QA-induced increases in caspase-1 activity and p53 protein levels, with no effect on QA-induced IκB-α degradation, NF -κB or AP-1 activation. Rats were pre-treated with intrastriatal infusion of Ac-YVAD-CHO (2-8 μg) before intrastriatal injection of QA (60 nmol). Striatal total proteins, genomic DNA, and nuclear proteins were isolated. The effects of Ac-YVAD-CHO on QA-induced caspase-1 activity, Internucleosomal DNA fragmentation, IκB-α degradation, NF-κB, and AP-1 activation, and increases in p53 protein levels were measured with enzyme assays, agarose gel electrophoresis, electrophoresis mobility shift assays, and Western blot analysis.[2] Stereotaxic drug administration Sprague-Dawley rats (300–350 g) were used. Rats were anesthetized with pentobarbital sodium (50 mg/kg). Stereotaxic drug administration was performed using a Kopf stereotaxic apparatus as described by Qin et al. To study the effects of a caspase-1 inhibitor on QA-induced internucleosomal DNA fragmentation, rats were either pretreated with an intrastriatal infusion of Ac-YVAD-CHO (2–8 µg) or Me2SO (2 µL) 10 min before instrastriatal injection of QA (60 nmol) and then killed 24 h after QA administration, or pre-treated with intrastriatal infusion of Ac-YVAD-CHO (4 µg) 10 min before instrastriatal injection of QA (60 nmol) and killed 12, 24, or 48 h after QA administration. Striatal genomic DNA was isolated and electrophoresed on a 2% agarose gel. To study the effect of a caspase-1 inhibitor on QA-induced increases in caspase-1 activity, rats were pre-treated with an intrastriatal infusion of Ac-YVAD-CHO (4 µg) or Me2SO (2 µL ) 10 min before intrastriatal injection of QA (60 nmol) and then killed 12 h after QA treatment. Striatal homogenates were used for assay of caspase-1 activity. To study the effect of a caspase-1 inhibitor on QA-induced increases in p53 proteins and NF-κB and AP-1 binding activities, rats were pre-treated with intrastriatal infusion of Ac-YVAD-CHO (4 µg) or Me2SO (2 µL) 10 min before intrastriatal injection of QA (60 nmol) and then killed 24 h after QA treatment. Total striatal proteins were extracted for Western blot analysis. Other animals were killed 12 h after QA treatment and nuclear proteins were isolated from the striatum for an electrophoresis mobility shift assay. |
| 参考文献 |
|
| 其他信息 |
In conclusion, we found that the caspase-1 inhibitor AcYVAD-CHO inhibited the QA-induced increase in p53 protein levels and internucleosomal DNA fragmentation, but had no effect on QA-induced IκB-α degradation and NF-κB activation. These results suggest that caspase-1 plays an important role in QA-induced p53 induction and apoptosis, but caspase-1 does not contribute to the QA-induced degradation of IκB-α or NF-κB activation. [2]
Caspase-1 activity was four times higher in SNAP-treated thymocytes at 8 and 12 h compared with untreated cells. Caspase-1 activity decreased thereafter. Both ZVAD-fmk and Ac-YVAD-cho inhibited NO-induced apoptosis when added up to 12 h after SNAP treatment. The inhibitory effect was lost after 16 h. PARP cleavage takes place after the amino acid sequence Ac-DEVD-cho. Originally this activity was attributed to caspase-3, but caspases-2,-4,-6,-7,-8, and -10, when added at high concentrations, can also cleave PARP. Because the rate of spontaneous apoptosis in our thymocyte cultures was 20%, it is not surprising that some (mild) PARP cleavage was found in untreated thymocytes. However, there was greater PARP cleavage after SNAP treatment, and it was blocked by Ac-YVAD-cho, even when added 8 h after SNAP treatment. Because neither caspase-3 nor caspase-9 was significantly activated during SNAP treatment, caspase-1 (or other caspases) was likely responsible for the cleavage of PARP in our cultures. [3] |
| 分子式 |
C25H36N4O10
|
|---|---|
| 分子量 |
552.57
|
| 精确质量 |
552.24314336
|
| 相关CAS号 |
Ac-YVAD-CHO;143313-51-3
|
| PubChem CID |
168007106
|
| 序列 |
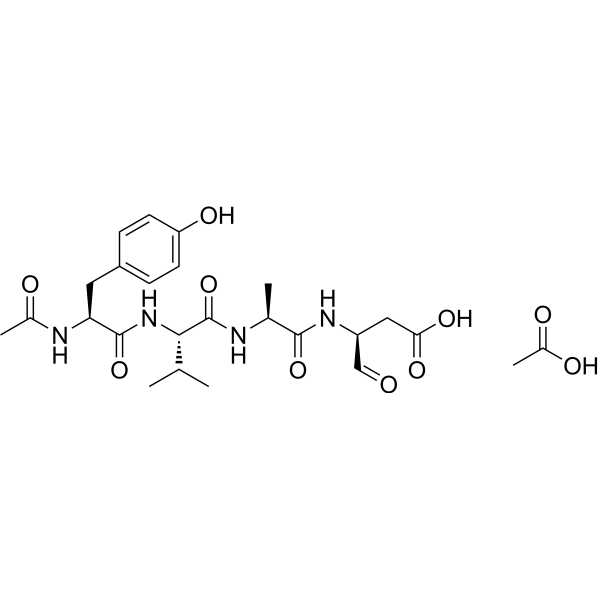
Ac-Tyr-Val-Ala-Asp-al.CH3CO2H; N-acetyl-L-tyrosyl-L-valyl-L-alanyl-L-aspart-1-al acetic acid;
|
| 短序列 |
YVAD
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| tPSA |
228 Ų
|
| 氢键供体(HBD)数目 |
7
|
| 氢键受体(HBA)数目 |
10
|
| 可旋转键数目(RBC) |
13
|
| 重原子数目 |
39
|
| 分子复杂度/Complexity |
811
|
| 定义原子立体中心数目 |
4
|
| SMILES |
C[C@@H](C(=O)N[C@@H](CC(=O)O)C=O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)C.CC(=O)O
|
| InChi Key |
BWQDODQMGYONOO-HZUAXBBDSA-N
|
| InChi Code |
InChI=1S/C23H32N4O8.C2H4O2/c1-12(2)20(23(35)24-13(3)21(33)26-16(11-28)10-19(31)32)27-22(34)18(25-14(4)29)9-15-5-7-17(30)8-6-15;1-2(3)4/h5-8,11-13,16,18,20,30H,9-10H2,1-4H3,(H,24,35)(H,25,29)(H,26,33)(H,27,34)(H,31,32);1H3,(H,3,4)/t13-,16-,18-,20-;/m0./s1
|
| 化学名 |
(3S)-3-[[(2S)-2-[[(2S)-2-[[(2S)-2-acetamido-3-(4-hydroxyphenyl)propanoyl]amino]-3-methylbutanoyl]amino]propanoyl]amino]-4-oxobutanoic acid;acetic acid
|
| 别名 |
Ac-YVAD-CHO acetate; Ac-YVAD-CHO (acetate); HY-120019A; Ac-YVAD-CHO (L-709049) acetate
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
|---|
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8097 mL | 9.0486 mL | 18.0973 mL | |
| 5 mM | 0.3619 mL | 1.8097 mL | 3.6195 mL | |
| 10 mM | 0.1810 mL | 0.9049 mL | 1.8097 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。