| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25g |
|
||
| 100g |
|
||
| 500g |
|
||
| Other Sizes |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Can be systemically absorbed after skin application, being found in the deeper layers of the stratum corneum as well as urine, plasma, and breast milk. The mean maximum plasma concentration detected after application of 2mg/cm2 sunscreen was 7ng/mL in women and 16ng/mL in men. Can be detected in urine in unchanged form. Naked rat skin. This was studied in a chamber experiment. Most of the material was found in the stripped skin; there was less in the stratum corneum, and least in the chamber. The approximate amounts found in the chamber were: after 6 hrs, 1.13 %; after 16 hrs, 11.4 %; and at 24 hrs 17,9 %. The figures for the horny layer and the strippings combined were, respectively, 31.4 %, 44.4 % and 45.7 % (percentages of applied doses). Solutions of 3% and 20 % of a.i. gave similar results. Eight healthy volunteers had small amounts of radioactive a.i. applied to the interscapular region. One group of 4 had the material applied under a watch glass; the other 4 had it applied on gauze, with occlusion in one case. Tests for absorption of a.i. were negative except for about 0.2 % in urine. The concentrations used were not stated. In a preliminary experiment, a capsule containing 100 mg of a.i. was taken orally. ... The cumulative excretion of 4methoxycinnamate in the urine over 24 hours was studied by GC/MS of the methyl ester derivative. (This method would also detect 4-hydroxycinnamic acid). Over 24 hours, 13.2 % of the amount ingested was recovered, equivalent to 21.5 % of the amount that would be expected if the a.i. were completely absorbed. In the main part of the experiment, an o/w cream containing 10 % a.i. was used. Applications of 2 grams of this material (= 200 mg a.i.) were made to the interscapular area of each of 5 male subjects, aged 29 to 46. The area of skin covered was 25x30 cm. After application, the area was covered with 3 layers of gauze, left in place for 12 hours. Blood was taken at times 0, 0.5, 1, 2, 3, 5, 7, and 24 hours. Urine was collected at 0, 1, 2, 3, 4, 5, 6, 7, 12, 24, 48, 72 and 96 hours. The control plasma samples showed a level equivalent to about 10 ng/ml before any application had been made. There was no evidence of any rise in plasma levels during the experiment. The urine showed a "physiological" level of 100 to 300 ng/ml. No significant increase in this amount was found in any sample. The authors conclude that very little, if any, of the compound was absorbed under the conditions of the experiment. The objective of this study was to determine the influence of vehicles on the penetration of octyl methoxycinnamate (OMC), as a UV absorber, to the stratum corneum by the stripping method. The experimental formulations consisted of a conventional o/w emulsion and multilamellar and small unilamellar liposomes (MLVs and SUVs) containing OMC. MLVs containing OMC were prepared by the fusion method and then converted to SUVs by probe sonication. Various formulations were then applied onto the midvolar forearms of six volunteers at a dose of 2 mg/sq cm. After determined timepoints, the stripping method was conducted whereby 22 tape strips were applied and subsequently divided into different stripping groups. The sunscreen agent was assessed by HPLC while the SPF (sun protection factor) of the formulations was determined in human volunteers in accordance with the Australian standard. Overall the results indicate that skin accumulation of OMC in MLVs was significantly greater than in the o/w emulsion and SUVs. Furthermore, SUV's penetration into the deeper skin layers was significantly greater than MLV's and that of a conventional o/w emulsion. Also, higher amounts of OMC were recovered from the upper layers of the stratum corneum than from the deeper layers in all the formulations tested. Finally, the SPF of the liposomes containing OMC was slightly greater than that of the control lotions at a similar concentration of OMC. In conclusion, the result of this study indicates that an MLV prepared by the fusion method could be a better vehicle for OMC as a sunscreen since it has a slightly better SPF compared to a conventional formulation and more remains in the stratum corneum, reducing its penetration to the deeper layers. For more Absorption, Distribution and Excretion (Complete) data for OCTINOXATE (19 total), please visit the HSDB record page. Metabolism / Metabolites Can undergo hepatic metabolism when systematically absorbed. Can be enzymatically degraded by lipases in the stratum corneum where esters undergo hydrolysis. Degrade into photoproducts when exposed to sunlight, which leads to a decrease in UV absorption efficiency. As a lipophilic substance, the a.i. is very likely to be metabolized; it is known in any case to be hydrolyzed by plasma esterases, although slowly. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
None Interactions Agricultural workers are encouraged to use sunscreen to decrease the risk of UV-related skin cancer. ... Previous studies have shown certain commercial sunscreens to be penetration enhancers. The focus of this project is to determine whether active ingredients in sunscreen formulations (i.e., the UV absorbing components and insect repellants for the sunscreen/bug repellant combinations) also act as dermal penetration enhancers for herbicides in vitro. The total percentages of 2,4-dichlorophenoxyacetic acid (2,4-D) penetrating through hairless mouse skin in 24 hr ranged from 54.9 +/- 4.7 for the no sunscreen control to 86.9 +/- 2.5 for padimate-O. Of the active ingredients tested (7.5% octyl methoxycinnamate, 7% octocrylene, 0.6% oxybenzone, 5% homosalate, 5% octyl salicylate, 8% padimate-o, 10% sulisobenzone, and 9.5% and 19% N,N-diethyl-m-toluamide (DEET)), all but octocrylene led to a significant increase in total 2,4-D penetration as compared to the control (P < 0.05), and only octocrylene and oxybenzone did not significantly decrease the corresponding lag time. Octyl salicylate (P < 0.01) and octyl methoxycinnimate (P < 0.05) significantly increased the 3H2O penetration across mouse skin, indicating physical damage to the stratum corneum. Additional studies demonstrated that the penetration enhancement seen across hairless mouse skin also occurred with human skin. Thus, the active ingredients of sunscreen formulations enhance dermal penetration of the moderately lipophilic herbicide 2,4-D. /The authors/sought to determine whether the effect of preapplication of a sun protection factor (SPF) 29 sunscreen (containing octyl methoxycinnamate, oxybenzone, and octyl salicylate) could prevent local UVB-induced suppression of contact hypersensitivity to dinitrochlorobenzene (DNCB). Nineteen subjects received either three minimal erythema doses of UVB daily on three consecutive days (UVB group) or sunscreen followed by this same dose of UVB irradiation (sunscreen plus UVB group) to a 16-sq cm area of the buttock. One day after completion of irradiation, DNCB was applied to this buttock site, and 2 weeks later, forearm challenge with four different concentrations of DNCB was performed. A control group of 10 subjects underwent DNCB testing as above, but with no prior exposure to UVB (no-UVB group). ... The UVB group had a reduced response rate to all challenge doses of DNCB (3.125, 6.25, and 8.8 ug), except for the highest dose (12.5 ug) compared with the no-UVB control group (Fisher's Exact test, P < or = 0.008), and compared with the sunscreen plus UVB group (P < or = 0.02). The no-UVB and sunscreen plus UVB groups showed no significant differences in response rates to any of the doses of DNCB tested (P > or = 0.53). ... These results indicate that application of a sunscreen with over ninefold greater protection than that needed to prevent erythema prior to localized UVB radiation prevents localized UVB-induced suppression of contact hypersensitivity... The influence of sucrose laureate and sucrose oleate on the in vivo percutaneous penetration of octyl methoxycinnamate (OMC) formulated in i) colloidal suspensions (nano-emulsions and nanocapsules), and ii) conventional o/w emulsions was evaluated. The results showed that nano-emulsions formulated with sucrose laureate exhibited the highest penetration in the stratum corneum compared to the other formulations. A two-fold increase in OMC skin deposition was observed with the nano-emulsion containing sucrose laureate when compared to the control. The data obtained suggest that the total amount of OMC detected in the stratum corneum and the penetration depth are strongly dependent upon the formulation's nature, the particle size, and the type of enhancer. Hairless mice were exposed to repeated doses of UV simulating the solar energy spectrum. After a rest period, 3 applications a week were made to an area of skin of 12-o-tetradecanoyl phorbol-13-acetate ... Suitable controls were used. The test group was completely protected by 50 % a.i., and 7.5 % gave an effect equivalent to reducing the insolation four-fold. It had been suggested that the a.i. could itself have been a promoter, but there was no evidence of this. For more Interactions (Complete) data for OCTINOXATE (7 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat oral >20 mL/kg b.w. LD50 Mouse oral >8 g/kg b.w. |
| 参考文献 | |
| 其他信息 |
2-ethylhexyl p-methoxycinnamate is a colorless to pale yellow viscous liquid. (NTP, 1992)
Octyl 4-methoxycinnamic acid is a cinnamate ester. Octinoxate is a cinnamate ester and common ingredient in sunscreen and other skin care products to minimize DNA photodamage. It was originally developed in 1950's as an organic UV-B filter that absorbs UV-B rays from sun. It is often combined with nanoparticles or other water-resistant liposomes in formulations to increase the localization at the epidermis and decrease the risk of percutaneous absorption. Its use in pharmaceutical and cosmetic formulations is approved by FDA. See also: Avobenzone; Octinoxate; Oxybenzone (component of); Octinoxate; Octocrylene (component of); Arbutin; octinoxate (component of) ... View More ... Drug Indication As an active ingredient in sunscreens and lip balms. Used for protection against damaging effects of sun rays. Mechanism of Action Absorbs UV-B (predominantly) and UV-A rays while accumulating in the outermost layer of the epidermis. Like any other photoprotective agents, octinoxate prevents the damage to cells and deoxyribonucleic acid (DNA) by reducing the p53 protein expression following UV exposure and also increases the skin's tolerability to UV rays. Diminish the penetration of ultraviolet (UV) light through the epidermis by absorbing UV radiation within a specific wavelength range. The amount and wavelength of UV radiation absorbed are affected by the molecular structure of the sunscreen agent. /Sunscreen agents, topical/ Radiation is absorbed by chemical sunscreens when the electron energy level of the drug is raised from its ground state to a higher energy level or excited state. Chromophore groups (C=C, C=O, O-N=O) with loosely held electrons are easily excited by radiation. Compounds which have several chromophore groups in optimal positions have high absorbance over a broad range of wavelengths. Chemical sunscreens are usually agents that absorb not less than 85% of UVB radiation (thus preventing burning) but may permit transmission of UVA radiation (thus allowing tanning). Some sunscreens may absorb wavelengths over a range that is slightly wider or narrower than that of UVB. All PABA derivatives absorb wavelengths of approximately 290-320 nm, benzophenone derivatives absorb wavelengths of approximately 250-360 nm, cinnamic acid derivatives absorb wavelengths of 280-320 nm, and salicylate derivatives and other miscellaneous chemical sunscreens absorb wavelengths of about 270-320 nm. The wavelength to which the skin is maximally sensitive had been accepted for many years to be 296.7 nm; however, recent evidence suggests that the most erythemogenic UVB wavelength may be slightly lower (e.g., somewhere in the range of 292-295 nm). In addition, of the stronger burning wavelengths that reach the earth's surface, most are approximately 310 nm. Therefore, sunscreens that maximally absorb UVB radiation near either of these wavelengths are particularly effective at preventing sunburn. Maximum absorbance occurs at about 290 nm for PABA, at about 295 nm for glyceryl-p-aminobenzoate, and at about 310 nm for the remaining PABA derivatives. Maximum absorbance occurs at 280-290 nm for benzophenone derivatives, at 310 nm for cinnamic acid derivatives with the exception of diethanolamine-p-methoxycinnamate which has its maximum absorbance at 290 nm, and at 300-305 nm for salicylate derivatives and other miscellaneous sunscreens. /Sunscreens/ Therapeutic Uses Ultraviolet /UVB/ screen /The authors/ tested the sun protection factor of a hydroquinone formulation (Lustra-Ultra, TaroPharma, Hawthorne, NY) containing avobenzone 3%, and octinoxate 7.5% according to the FDA Sunscreen Monograph on 20 volunteer subjects. We also determined the UVR absorbance spectrum of the preparation. ... The mean sun protection factor (SPF) of 21.7 satisfied labeling requirements for SPF 20. The formulation exhibited strongest photoprotection near the wavelengths of peak sun burning effectiveness in the UVB region and maintains significant UVR absorbance through the entire UVA region. Avobenzone 3% and octinoxate 7.5% provide broad spectrum UV protection. Incorporating these sunscreens into a hydroquinone preparation simplifies the treatment regimen while providing significant photoprotection for patients being treated for dyschromia. Sunscreens capable of inhibiting erythema are assumed to protect against UV-induced carcinogenesis as well. However, the correlation between inflammation and carcinogenesis is uncertain, and the prevention of UV-induced erythema might in fact be biologically irrelevant as an indicator of protection against UV-induced skin cancer. Ultraviolet-B radiation promotes cutaneous immunosuppression by the release of immunoregulatory cytokines and by depletion of Langerhans cells. /The authors/ investigated the ability of two different sunscreens to inhibit UVB-induced expression of epidermal interleukin (IL)-10 and depletion of Langerhans cells. Chemical and physical sunscreens were applied to the forearms of volunteers 15 min prior to 4 minimal erythemal doses of UVB exposure. Suction blisters were induced 24 hr after irradiation, and RNA was extracted from the blister roofs. Reverse transcription polymerase chain reaction was performed using primers for IL-10 and CD1a. A chemical sunscreen containing octyl methoxycinnamate (12 sun protection factor (SPF)) and a physical sunscreen containing zinc oxide (16 SPF) were assayed: UVB-induced IL-10 mRNA expression was nearly totally inhibited by both sunscreens (median protection for chemical and physical sunscreens was 95% and 78%, respectively), whereas UVB-induced Langerhans cell depletion was partially prevented (47% and 50% for chemical and physical sunscreens, respectively). Langerhans cell protection by sunscreens was confirmed by estimation of cell density after ATPase staining. In contrast, both sunscreens effectively prevented the induction of UVB-induced erythema. /The authors/ believe this to be the first demonstration that sunscreens can prevent the induction of cutaneous mediators of immunosuppression, and that the results indicate that the immunoprotection offered by the sunscreens is significantly lower than their ability to prevent erythema. Daily use of a sunscreen with a high SPF (greater than 15) on usually exposed skin is recommended for residents of areas of high ... /solar radiation/ who work outdoors or ... /enjoy/ regular outdoor recreation. Daily use of a sunscreen can reduce the cumulative ... /solar/ exposure that causes actinic keratoses and squamous-cell carcinoma. Sunscreen agents are indicated for the prevention of sunburn. In addition to limiting the skin's exposure to the sun, using sunscreen agents regularly when in the sun may help reduce long-term sun damage such as premature aging of the skin and skin cancer. /Sunscreen agents, topical; Included in US product labeling/ Drug Warnings The manufacturers of sunscreen preparations with propellants warn that concentrating and subsequently inhaling the fumes from these preparations may be harmful or fatal. /Propellants/ Because the absorptive characteristics of skin of children younger than 6 months of age may differ from those of adults and because the immaturity of metabolic and excretory pathways of these children may limit their ability to eliminate any percutaneously absorbed sunscreen agent, sunscreen products should be used in children younger than 6 months of age only as directed by a clinician. It is possible that the characteristics of geriatric skin also differ from those of skin in younger adults, but these characteristics and the need for special considerations regarding use of sunscreen preparations in this age group are poorly understood. /Sunscreens/ Little information is available regarding the safety of chronic sunscreen usage, but commercially available physical and chemical sunscreens appear to have a low incidence of adverse effects. Derivatives of PABA, benzophenone, cinnamic acid, and salicylate and 2-phenylbenzimidazole-5-sulfonic acid have caused skin irritation including burning, stinging, pruritus, and erythema on rare occasions. /Sunscreens/ Sunscreens should not be used as a means of extending the duration of solar exposure, such as prolonging sunbathing, and should not be used as a substitute for clothing on usually unexposed sites, such as the trunk and buttocks. /Sunscreens/ For more Drug Warnings (Complete) data for OCTINOXATE (11 total), please visit the HSDB record page. Pharmacodynamics Acts as a photoprotective agent that protects the skin by preventing and minimizing the damaging effects of ultraviolet (UV) rays of natural light. The cellular effects of UV irradiation include DNA damage, cell cycle arrest, immunological depression, apoptosis, and transcriptional changes. |
| 分子式 |
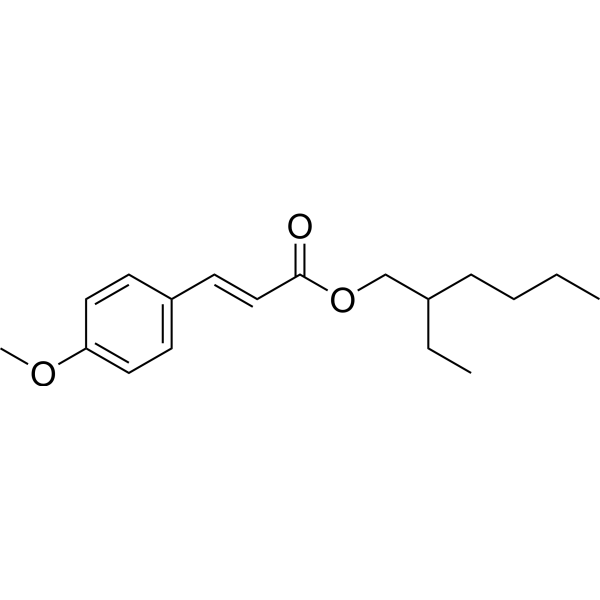
C18H26O3
|
|---|---|
| 分子量 |
290.40
|
| 精确质量 |
290.188
|
| CAS号 |
83834-59-7
|
| PubChem CID |
5355130
|
| 外观&性状 |
Pale yellow liquid
Colorless to light yellow viscous liquid |
| 密度 |
1.0±0.1 g/cm3
|
| 沸点 |
405.3±20.0 °C at 760 mmHg
|
| 熔点 |
less than -13 °F (NTP, 1992)
-25°C -68.3 °C using OECD Guideline 102 (Melting point/Melting Range) |
| 闪点 |
171.6±16.4 °C
|
| 蒸汽压 |
0.0±0.9 mmHg at 25°C
|
| 折射率 |
1.515
|
| LogP |
5.66
|
| tPSA |
35.53
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
10
|
| 重原子数目 |
21
|
| 分子复杂度/Complexity |
304
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CCCCC(CC)COC(=O)/C=C/C1=CC=C(C=C1)OC
|
| InChi Key |
YBGZDTIWKVFICR-JLHYYAGUSA-N
|
| InChi Code |
InChI=1S/C18H26O3/c1-4-6-7-15(5-2)14-21-18(19)13-10-16-8-11-17(20-3)12-9-16/h8-13,15H,4-7,14H2,1-3H3/b13-10+
|
| 化学名 |
2-ethylhexyl (E)-3-(4-methoxyphenyl)prop-2-enoate
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
Typically soluble in DMSO (e.g. 10 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.4435 mL | 17.2176 mL | 34.4353 mL | |
| 5 mM | 0.6887 mL | 3.4435 mL | 6.8871 mL | |
| 10 mM | 0.3444 mL | 1.7218 mL | 3.4435 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。