| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
B-Raf
|
|---|---|
| 体外研究 (In Vitro) |
PF-07799933是一种脑渗透、选择性、泛突变的BRAF抑制剂。PF-07799933在体外抑制brafv600e突变单体、BRAF II/ iii类突变二聚体以及诱导突变brafv600e二聚体的治疗获得性遗传改变驱动的细胞中的pERK。此外,PF-07799933在含有BRAFV600E + p61剪接变体或BRAFV600E + NRASQ61K的细胞中破坏内源性突变BRAF:野生型craf二聚体,诱导突变BRAF二聚体。然而,PF-07799933在体外BRAF野生型细胞中不破坏pERK,也不破坏BRAF野生型:craft野生型二聚体。[1]
PF-07799933破坏突变BRAF二聚体,在临床前克服多种BRAF突变在BRAF野生型细胞中,与泛raf二聚体抑制剂相比,PF-07799933没有表现出pERK抑制作用,并且比encorafenib更少地激活pERK。等温稳定性位移剂量反应试验(ITDR)显示,在A375 BRAFV600E突变黑色素瘤细胞裂解物中,PF-07799933结合BRAFV600E的亲和力比野生型CRAF蛋白高10倍。 |
| 体内研究 (In Vivo) |
PF-07799933在体内和脑内具有广泛的抗肿瘤活性,可作为单药治疗BRAFV600E和非v600突变蛋白,以及治疗获得性BRAF p61剪接变体联合binimetinib治疗BRAFV600E。[1]
在含有BRAFV600E (I类)突变的小鼠异种移植物皮下或颅内植入中,PF-07799933单药治疗比encorafenib + binimetinib带来更深的退化。PF-07799933治疗也导致皮下植入BRAFG469A (II类)突变型NSCLC、BRAFK601E (II类)突变型黑色素瘤和BRAF indel突变型胰腺癌肿瘤的消退。相比之下,plixorafenib对BRAFV600E-和brafg469a突变模型的疗效较差,这与较少的体外pERK抑制和已知的体内代谢脆弱性相一致。同样,与较少的体外pERK抑制一致,exarafenib对brafv600e突变模型的活性较低。对于plixorafenib和exarafenib,我们确认动物暴露量与人类接近。因此,观察到的相对于PF-07799933±比尼美替尼的疗效下降不是由于相对于临床可达到的人体剂量的暴露减少。在含有获得性BRAF p61剪接变体的brafv600突变黑色素瘤患者衍生异种移植瘤(PDX)模型中,恩可非尼+比尼美替尼联合治疗并没有提高比尼美替尼单药治疗的最低抗肿瘤活性。然而,单药PF-07799933表现出更强的活性,与binimetinib联用进一步增强了疗效,导致肿瘤消退,同时保持良好的耐受性,没有体重减轻。[1] |
| 酶活实验 |
将Braf KDS: encorafenib复合物与储层溶液(含18% PEG3350、0.2 mol/L Na2SO4、0.1 mol/L磷酸钠钾、pH 6.6)等量混合,采用悬垂气相扩散法进行共结晶。对于Braf-KDL: PF-07799933络合结晶,储层溶液中含有17% PEG5000MME、1% PEG6000、醋酸钠、0.2 mol/L NaCl和5% Tacsimate。收集两种晶体并在含有20% (v/v)甘油作为冷冻保护剂的储层溶液中快速冷冻在液氮中。[1]
|
| 细胞实验 |
等温稳定性偏移剂量反应试验[1]
胰蛋白酶化的A375细胞微球在PBS中重悬,用DMSO或PF-07799933(浓度范围为0.122至2,000 nmol/L)在37℃下处理30分钟。使用PTC-200热循环仪将细胞在PCR板中加热至50°C 3分钟,然后在4°C的摆动桶离心机中快速旋转4700 rpm 30秒。在加入非变性缓冲液[10-mmol/L Na2PO4、1.8-mmol/L KH2PO4 (pH 7.4)、137-mmol/L NaCl、2.7-mmol/L KCl、1-mmol/L CaCl2、10-mmol/L MgCl2、0.02% n-十二烷基β- d -麦糖苷、2×完全蛋白酶抑制剂和2%磷酸酶抑制剂混合物2和3]之前,在液氮中冷冻解冻3次细胞。 免疫共沉淀和免疫印迹[1] 对于内源性RAF蛋白的共免疫沉淀,将MEL21514或A375-NRASQ61K细胞以1.5 × 107个细胞/ 150-mm培养皿的方式进行免疫沉淀。细胞在37℃,5% CO2, DMSO对照或指定浓度的encorafenib或PF-07799933条件下孵育1小时。细胞裂解液以每盘1 mL的镁裂解缓冲液[25 mmol/L 4-(2-羟乙基)-1-哌嗪乙磺酸pH 7.5, 75 mmol/L NaCl, 5 mmol/L MgCl2, 5%甘油和0.1% NP-40]中添加HALT蛋白酶/磷酸酶抑制剂混合物。 |
| 动物实验 |
Efficacy studies with the CTG-1441 and CTG-0362 PDX models were performed at a CRO company. The MEL21514 PDX was generated from a biopsy provided by a female Hispanic patient with melanoma (51 years of age) following a relapse from Braftovi and Mektovi treatment (MT Group). BRAFV600E mutation and p61 splice variant expression in the MEL21514 PDX tumor were confirmed by whole-exome sequencing and RNA sequencing, respectively. For cell line development, MEL21514 PDX tumor samples were dissociated using the Miltenyi Biotec Human Tumor Dissociation Kit in combination with the gentleMACS Octo Dissociator according to the suggested hard tumor protocol. The dissociated cell suspension was then magnetically labeled with the Miltenyi Mouse Cell Depletion Kit and separated using the MACS Magnetic Separator for the enrichment of human tumor cells. Isolated MEL21514 tumor cells were pooled and established in culture using Renaissance Essential Tumor Medium/RETM supplemented with 10% fetal bovine serum/FBS, penicillin/streptomycin and cholera toxin. Following 10 passages in RETM, MEL21514 cells were grown in RPMI, supplemented with 10% FBS and 1-mmol/L sodium pyruvate. Cell lines and PDXs were authenticated by short tandem repeat profiling and regularly evaluated for Mycoplasma and murine viruses.
All mice were obtained at 6 to 8 weeks of age, housed in groups of 5, and allowed a 1-week acclimation period before cancer cell inoculation. Food, water, temperature, and humidity were maintained per Pharmacology Testing Facility performance standards in accordance with the 2011 Guide for the Care and Use of Laboratory Animals (NRC) and AAALAC-International. For subcutaneous xenografts, each cell line (5 × 106 cells) or PDX (cell suspension prepared with Miltenyi gentleMACS) was injected subcutaneously into the right flank of female Foxn1nu mice and allowed to grow to approximately 200 mm3 prior to randomization by tumor size into dosing groups of eight animals. Body weight and subcutaneous tumor volume [determined by the formula (length × width2)/2] were recorded twice weekly. For intracranial xenografts, the A375-luciferase cell line (10,000 cells) was injected 2 mm lateral to the bregma at the bone suture line of female Foxn1nu mice, with randomization after 7 days into dosing groups of ten animals based on tumor burden measured by total luminescence flux (photons/second) with an IVIS Spectrum In Vivo Imaging System. Body weight and total flux were recorded twice weekly, with average total flux plotted against the day posttumor implantation.
|
| 毒性/毒理 (Toxicokinetics/TK) |
PF-07799933 was well-tolerated as monotherapy or combination. There were no DLTs and the MTD was not reached; ≥1 treatment-emergent AE (TEAE) with monotherapy and combination occurred in 94% and 100% of patients, respectively, and was grade ≥3 in 28% and 44%, respectively. TEAEs reported in ≥3 patients regardless of attribution are shown in Supplementary Table S4. The most common TEAEs for monotherapy were fatigue (any grade 44%/grade ≥3 0%), headache (28%/0%), vision blurred [22%/6% (a single patient with grade 3)], and lipase increased (16%/0%). The most common TEAEs for combinations were peripheral edema (33%/0%), acneiform rash, diarrhea, and fatigue (each 28%/0%). A single patient treated with PF-07799933 at 450 mg BID monotherapy had their dose reduced for AEs of vision blurred, peripheral sensory neuropathy, myalgia, fatigue, and decreased appetite. 6/8 AEs of blurred vision resolved without dose modification, and there were no abnormal findings reported on repeat ophthalmic examinations. No patient discontinued treatment for a study drug-related AE.[1]
|
| 参考文献 |
[1]. A Next-Generation BRAF Inhibitor Overcomes Resistance to BRAF Inhibition in Patients with BRAF-Mutant Cancers Using Pharmacokinetics-Informed Dose Escalation. Cancer Discov . 2024 Sep 4;14(9):1599-1611.
|
| 其他信息 |
RAF inhibitors have transformed treatment for patients with BRAFV600-mutant cancers, but clinical benefit is limited by adaptive induction of ERK signaling, genetic alterations that induce BRAFV600 dimerization, and poor brain penetration. Next-generation pan-RAF dimer inhibitors are limited by a narrow therapeutic index. PF-07799933 (ARRY-440) is a brain-penetrant, selective, pan-mutant BRAF inhibitor. PF-07799933 inhibited signaling in vitro, disrupted endogenous mutant-BRAF:wild-type-CRAF dimers, and spared wild-type ERK signaling. PF-07799933 ± binimetinib inhibited growth of mouse xenograft tumors driven by mutant BRAF that functions as dimers and by BRAFV600E with acquired resistance to current RAF inhibitors. We treated patients with treatment-refractory BRAF-mutant solid tumors in a first-in-human clinical trial (NCT05355701) that utilized a novel, flexible, pharmacokinetics-informed dose escalation design that allowed rapid achievement of PF-07799933 efficacious concentrations. PF-07799933 ± binimetinib was well-tolerated and resulted in multiple confirmed responses, systemically and in the brain, in patients with BRAF-mutant cancer who were refractory to approved RAF inhibitors. Significance: PF-07799933 treatment was associated with antitumor activity against BRAFV600- and non-V600-mutant cancers preclinically and in treatment-refractory patients, and PF-07799933 could be safely combined with a MEK inhibitor. The novel, rapid pharmacokinetics (PK)-informed dose escalation design provides a new paradigm for accelerating the testing of next-generation targeted therapies early in clinical development.[1]
PF-07799933 (ARRY-440) is a next-generation, selective pan-mutant BRAF inhibitor that is not a pan-RAF inhibitor, does not possess the metabolic liability of plixorafenib, can be combined with MEK inhibitors, and is brain-penetrant. Given the significant unmet need faced by patients with BRAF-mutant cancers after the failure of available treatments, we aimed to implement a data-informed dose escalation approach in the first-in-human phase 1 trial to achieve therapeutic exposures in less time and with fewer patients overall.[1] |
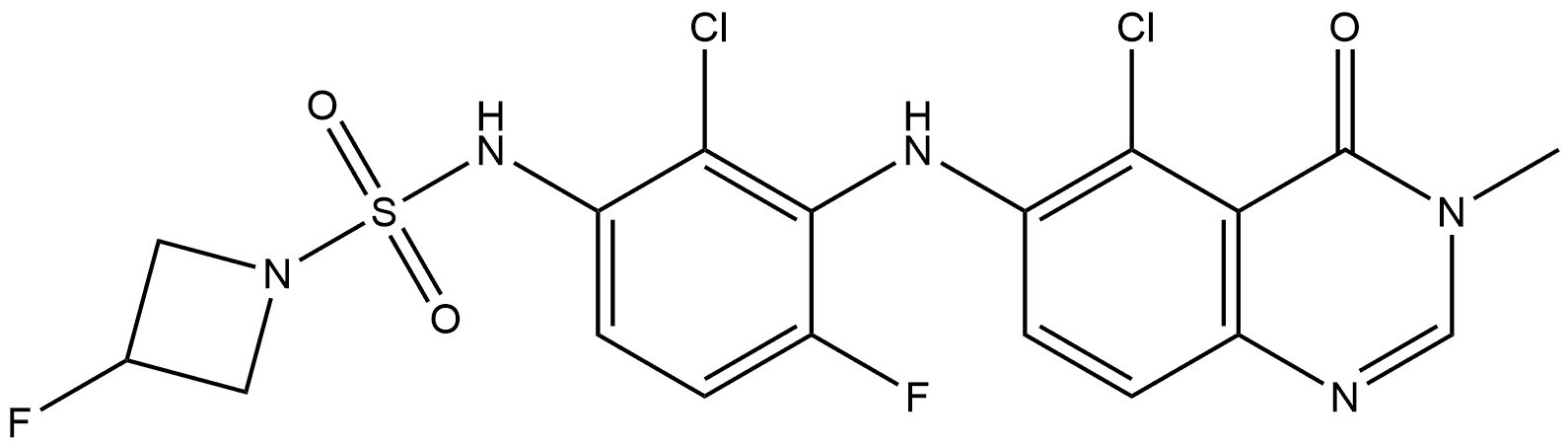
| 分子式 |
C18H15CL2F2N5O3S
|
|---|---|
| 分子量 |
490.31
|
| 精确质量 |
489.0240722
|
| CAS号 |
2754408-94-9
|
| PubChem CID |
165150001
|
| 外观&性状 |
White to light yellow solid powder
|
| 沸点 |
585.0±60.0 °C(Predicted)
|
| 熔点 |
1.72±0.1 g/cm3(Temp: 20 °C; Press: 760 Torr)(Predicted)
|
| LogP |
2.8
|
| tPSA |
103Ų
|
| SMILES |
CN1C=NC2=C(C1=O)C(=C(C=C2)NC3=C(C=CC(=C3Cl)NS(=O)(=O)N4CC(C4)F)F)Cl
|
| InChi Key |
SHENFUUACGRLOZ-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C18H15Cl2F2N5O3S/c1-26-8-23-11-4-5-12(15(19)14(11)18(26)28)24-17-10(22)2-3-13(16(17)20)25-31(29,30)27-6-9(21)7-27/h2-5,8-9,24-25H,6-7H2,1H3
|
| 化学名 |
N-[2-chloro-3-[(5-chloro-3-methyl-4-oxoquinazolin-6-yl)amino]-4-fluorophenyl]-3-fluoroazetidine-1-sulfonamide
|
| 别名 |
2754408-94-9; PF07799933; Claturafenib (USAN); PF-07799933; SCHEMBL25280690; N-(2-chloro-3-((5-chloro-3-methyl-4-oxo-3,4-dihydroquinazolin-6-yl)amino)-4-fluorophenyl)-3-fluoroazetidine-1-sulfonamide
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0395 mL | 10.1976 mL | 20.3953 mL | |
| 5 mM | 0.4079 mL | 2.0395 mL | 4.0791 mL | |
| 10 mM | 0.2040 mL | 1.0198 mL | 2.0395 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。