| 规格 | 价格 | ||
|---|---|---|---|
| 500mg | |||
| 1g | |||
| Other Sizes |
| 靶点 |
Endogenous Metabolite
|
|---|---|
| 体外研究 (In Vitro) |
原卟啉 IX 二钠 (3.6 nmol/g 组织) 在光照下可诱导正常大鼠结肠粘膜坏死[4]。原卟啉 IX 二钠 (6.6 nmol/g 肿瘤) 可延缓大鼠肿瘤照射后的肿瘤生长[4]。原卟啉 IX 二钠 (0-25 μM, 2 小时) 可迅速导致 Hela 细胞核 G4 水平升高[5]。
Olivo等人报告称,经尿道切除更高级别和更高阶段的膀胱肿瘤后,ALA滴注后可产生和积累更高水平的原卟啉IX。他们的发现支持我们的结果。Miyake及其同事表明,癌症尿路上皮中铁螯合酶的低表达或分子缺陷与细胞内原卟啉IX的积累有关。Hagiya及其同事报告称,肽转运蛋白1和人ATP结合盒转运蛋白2的表达水平在ALA诱导的原卟啉IX积累中起着关键作用。Ogino等人报道了铁螯合酶抑制剂和人ATP结合盒转运蛋白2抑制剂刺激ALA诱导的T24细胞中原卟啉IX的积累。多个因素似乎与高级别肿瘤中原卟啉IX积累增加的机制有关。哪个因素起主导作用是未来研究的课题。[1] 哺乳动物卟啉是由δ-氨基酮戊酸(ALA)通过几个连续的酶促反应生成原卟啉IX(PpIX),后者进一步与铁阳离子络合,产生血红素或其衍生物氯化血红素[5]。 |
| 体内研究 (In Vivo) |
给予氨基乙酰丙酸(300 mg/kg,单次静脉注射)三小时后,在肝脏和肠道中发现原卟啉 IX 二钠的浓度最高(约 6.3 nmol/g 组织),其次是主动脉(4.3 nmol/g 组织)和食道(2.1 nmol/g 组织)[4]。
有限的穿透深度显著限制了局部使用δ(5)-氨基酮戊酸(ALA)对结节性基底细胞癌(BCC)的光动力治疗。为了证明口服ALA诱导BCC内源性原卟啉IX(PpIX)产生的安全性和有效性,13名BCC患者按照剂量递增方案摄入了ALA。所有剂量范围(10、20或40mg/kg单次剂量)均导致人体皮肤和基底细胞癌中形成Protoporphyrin IX/PpIX,可通过体内荧光分光光度法测量。摄入ALA后1至3小时,肿瘤中的PpIX荧光在正常邻近皮肤之前达到峰值。离体标本的总荧光成像显示,仅在40mg/kg剂量下,肿瘤中的PpIX荧光比正常皮肤更大。荧光显微镜证实了这一发现,仅在给予40mg/kg的ALA后,所有BCC亚型都显示出明显的全层PpIX荧光。副作用是剂量依赖性和自限性的。在20和40mg/kg剂量下,光敏性持续时间小于24小时,恶心与皮肤PpIX荧光峰值一致。40mg/kg ALA后,血清肝酶水平在24小时内升至最高值,然后在1-3周内消失。两名患者出现短暂性胆红素尿。[3] 在这项研究中,研究了5-氨基酮戊酸(ALA)在大鼠体内的生物分布和原卟啉IX(PpIX)的积累。两组21只WAG/Rij大鼠口服或静脉注射200mg/kg ALA。六只老鼠作为对照。在ALA给药后1、2、3、4、6、12和24小时,测量18种组织和液体中的ALA和卟啉浓度。测量肝酶和肾功能测试以确定ALA毒性。在两组中,肾脏、膀胱和尿液中的ALA浓度最高。口服给药后,十二指肠吸出物和空肠中也发现了高浓度。两个治疗组均出现轻度、短暂的肌酐升高。卟啉,尤其是PpIX,主要在十二指肠吸出物、空肠、肝脏和肾脏中积累(>10 nmol/g组织),在食管、胃、结肠、脾脏、膀胱、心脏、肺和神经中积累较少(2-10 nmol/g组),在血浆、肌肉、脂肪、皮肤和脑中仅少量积累(<2 nmol/g)。卟啉的原位合成而不是肠肝循环有助于Protoporphyrin IX/PpIX的积累。共聚焦激光扫描显微镜显示上皮层中的选择性卟啉荧光。口服和静脉注射ALA后,卟啉的峰值水平和总产量相等。 总之:200 mg/kg ALA给药后1至6小时,除肌肉、脂肪、皮肤和脑外,所有组织中都会积累光敏浓度的Protoporphyrin IX/PpIX。了解时间-浓度关系应有助于选择ALA光动力疗法的剂量、给药途径和时间[4]。 |
| 细胞实验 |
原卟啉IX在膀胱癌症脱落细胞中的光动力学检测[1]
在适当的设置下(激发波长405 nm,发射波长550-700 nm,增益160),使用分光光度计测试每个腔室中ALA处理和ALA未处理的尿液沉积物的原卟啉IX荧光。当样品强度超出范围时,调整分光光度计的增益。用来自膀胱癌症患者的ALA处理的样品的光谱在635nm处显示出峰值,而ALA对照样品的光谱没有显示出这样的峰值(图2a)。对照组患者的样本中未检测到峰值(图2b)。 原卟啉IX在脱落的膀胱癌症细胞中的评价[1] 为了评估635nm处的峰值,计算了ALA处理和ALA未处理样品的强度差异。该分析的曲线下面积、敏感性和特异性分别为0.68、60%和65%。如上所述,当样品的强度超出测量范围时,调整增益。因此,将计算出的差异除以635nm处未经ALA处理的样品的强度,以调整不同增益设置的数据。调整后的曲线下面积、敏感性和特异性分别为0.74、73%和63%。由于ALA处理和未处理的样品之间存在差异,一些在635nm处没有峰值的对照病例被诊断为阳性;在这些样品中,在550nm至700nm的范围内检测到635nm处的差异(图3b)。为了解决这个问题,从ALA处理和ALA未处理样品在635 nm处的强度差中减去ALA处理与ALA未治疗样品在600 nm下的强度差(图3a和b)。之后,将调整后的差值除以ALA未处理样品在635nm处的强度。这种调整后的变化率用于诊断膀胱癌症(ALA诱导荧光细胞学方法)。 |
| 动物实验 |
Porphyrin analysis [4]
The analysis was carried out according to Chisolm and Brown with the following modifications: the tissues were suspended in sterile water ( 1: 10 wt./vol.) and homogenized in a tissue grinder [ 2 11. To 100 ~1 of this homogenate the following were added: 700 p,l HCl 2 mmol/l and 800 p,l ethyl-acetate/glacial acetic acid (3: 1). After 10 min centrifugation at 18OOg, the ethyl-acetate phase with some protein is removed, and after a second centrifugation for 5 min at 1800g the HCl phase is measured in an LS 5B fluorescence spectrometer using a red-sensitive photomultiplier at an excitation wavelength of 410 nm and an emission wavelength of 650 nm. Although porphyrins are commonly determined by emission at 60 1 nm, under the conditions described, Protoporphyrin IX/PpIX has also a specific emission at 650 nm, which is about 80% of the emission at 601 nm and can be easily detected using a redsensitive photomultiplier. In our experience, bile acids in duodenal aspirates, and to a lesser extent other tissues, contain fluorophores with high emission at lower wavelength, which gave rise to interference in porphyrin quantitation at 601 nm. Emission at 650 nm in a direct extraction assay as described above was found to be proportional to Protoporphyrin IX/PpIX quantification, determined by a limited number of HPLC porphyrin separations. Porphyrin standards were analysed separately for their actual concentration using UV spectroscopy and the molar extinction coefficient ( .sdo7 = 0.275 L mol-’ cm-‘). Recovery of porphyrins was checked by adding standard Protoporphyrin IX/PpIX to the samples. A recovery between 90 and 100 was achieved. |
| 药代性质 (ADME/PK) |
This study focalized on three PSs that are used clinically (PpIX and PF) or for in vivo experiments (PPa). Our team proposed PPa coupled to folic acid to treat ovarian metastases by PDT (Patent WO/2019/016397).
By analyzing the photophysical properties of these three PSs in different conditions, we highlighted the fact that each PS is unique and reacts very differently depending on its chemical structure and concentration. If the change of the medium polarity does not greatly affect the UV-visible absorption spectrum of PF, there is a drastic change for PpIX and PPa. In the literature, it is often claimed that PpIX should be excited at 630 nm in vitro or in vivo. This excitation wavelength is based on the absorption spectrum in ethanol. In FBS and PBS, which are aqueous media more similar to physiological media, the QI band is located at 641 nm. Depending on the localization of the PS in the cells, the local viscosity can be very different. We could also observe that modifying the solvent viscosity did not greatly affect the maximal wavelengths of absorption of QI in PpIX and PF but it was blue-shifted for PPa for 10 nm (from 678 nm to 668 nm). Temperature change slightly affected the UV-visible absorption spectra of PpIX and PF but drastically modified the UV-visible absorption of PPa in the range of 10 to 40 °C. Finally, modifying pH also induced a shift of QI band for PPa of 25 nm (from 704 nm to 679 nm). Perhaps the most interesting results are the ΦΔ obtained in different solvents. Depending on the solvent, the values were totally different. In toluene, we could not detect any 1O2 whereas the ΦΔ were quite good for PpIX and PPa 0.68 and 0.49, respectively. In EtOH, the ΦΔ was 0.92, 0.53, and 0.80 for PpIX, PPa, and PF, respectively. If we switched to D2O, we could not detect any 1O2 of PpIX or PPa and the ΦΔ was 0.15 for PF. Moreover, in real-life applications, the PS is ideally in a cellular context. The presence of protein, lipid, and other biomolecules molecules will also affect the photophysics of the PS. This raised the question of what type of experiments and which solvent should be used in the solution when performing in vitro studies.[2] |
| 毒性/毒理 (Toxicokinetics/TK) |
71484 rat LD50 intravenous 240 mg/kg Drugs in Japan, 6(729), 1982
71484 mouse LD50 oral >5 gm/kg Drugs in Japan, -(1192), 1995 71484 mouse LD50 intraperitoneal 1029 mg/kg Drugs in Japan, 6(729), 1982 71484 mouse LD50 subcutaneous 1147 mg/kg Drugs in Japan, 6(729), 1982 71484 mouse LD50 intravenous 484 mg/kg Drugs in Japan, 6(729), 1982 |
| 参考文献 |
|
| 其他信息 |
Protoporphyrin is a cyclic tetrapyrrole that consists of porphyrin bearing four methyl substituents at positions 3, 8, 13 and 17, two vinyl substituents at positions 7 and 12 and two 2-carboxyethyl substituents at positions 2 and 18. The parent of the class of protoporphyrins. It has a role as a photosensitizing agent, a metabolite, an Escherichia coli metabolite and a mouse metabolite. It is a conjugate acid of a protoporphyrinate and a protoporphyrin(2-).
Protoporphyrin IX is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). protoporphyrin IX has been reported in Homo sapiens and Turdus merula with data available. Protoporphyrin IX is a tetrapyrrole containing 4 methyl, 2 propionic and 2 vinyl side chains that is a metabolic precursor for hemes, cytochrome c and chlorophyll. Protoporphyrin IX is produced by oxidation of the methylene bridge of protoporphyrinogen by the enzyme protoporphyrinogen oxidase. Protoporphyrin IX is a metabolite found in or produced by Saccharomyces cerevisiae. Background: We evaluated the feasibility of photodynamic diagnosis of bladder cancer by spectrophotometric analysis of voided urine samples after extracorporeal treatment with 5-aminolevulinic acid (ALA). Methods: Sixty-one patients with bladder cancer, confirmed histologically after the transurethral resection of a bladder tumor, were recruited as the bladder cancer group, and 50 outpatients without history of urothelial carcinoma or cancer-related findings were recruited as the control group. Half of the voided urine sample was incubated with ALA (ALA-treated sample), and the rest was incubated without treatment (ALA-untreated sample). For detecting cellular protoporphyrin IX levels, intensity of the samples at the excitation wavelength of 405 nm was measured using a spectrophotometer. The difference between the intensity of the ALA-treated and ALA-untreated samples at 635 nm was calculated. Results: The differences in the bladder cancer group were significantly greater than those in the control group (p < 0.001). These differences were also significantly greater in patients with high-grade tumors than in those with low-grade tumors (p = 0.004), and also in patients with invasive bladder cancer than in those with noninvasive bladder cancer (p = 0.007). The area under the curve was 0.84. Sensitivity and specificity of the method were 82% and 80%, respectively. Conclusions: We demonstrated that protoporphyrin IX levels in urinary cells treated with ALA could be quantitatively detected by spectrophotometer in patients with bladder cancer. Therefore, this cancer detection system has a potential for clinical use.[1] Photodynamic therapy (PDT) is an innovative treatment of malignant or diseased tissues. The effectiveness of PDT depends on light dosimetry, oxygen availability, and properties of the photosensitizer (PS). Depending on the medium, photophysical properties of the PS can change leading to increase or decrease in fluorescence emission and formation of reactive oxygen species (ROS) especially singlet oxygen (1O2). In this study, the influence of solvent polarity, viscosity, concentration, temperature, and pH medium on the photophysical properties of protoporphyrin IX, pyropheophorbide-a, and Photofrin® were investigated by UV-visible absorption, fluorescence emission, singlet oxygen emission, and time-resolved fluorescence spectroscopies.[2] Background: G-quadruplexes (G4s) are unique noncanonical nucleic acid secondary structures, which have been proposed to physically interact with transcription factors and chromatin remodelers to regulate cell type-specific transcriptome and shape chromatin landscapes. Results: Based on the direct interaction between G4 and natural porphyrins, we establish genome-wide approaches to profile where the iron-liganded porphyrin hemin can bind in the chromatin. Hemin promotes genome-wide G4 formation, impairs transcription initiation, and alters chromatin landscapes, including decreased H3K27ac and H3K4me3 modifications at promoters. Interestingly, G4 status is not involved in the canonical hemin-BACH1-NRF2-mediated enhancer activation process, highlighting an unprecedented G4-dependent mechanism for metabolic regulation of transcription. Furthermore, hemin treatment induces specific gene expression profiles in hepatocytes, underscoring the in vivo potential for metabolic control of gene transcription by porphyrins. Conclusions: These studies demonstrate that G4 functions as a sensor for natural porphyrin metabolites in cells, revealing a G4-dependent mechanism for metabolic regulation of gene transcription and chromatin landscapes, which will deepen our knowledge of G4 biology and the contribution of cellular metabolites to gene regulation.[5] |
| 分子式 |
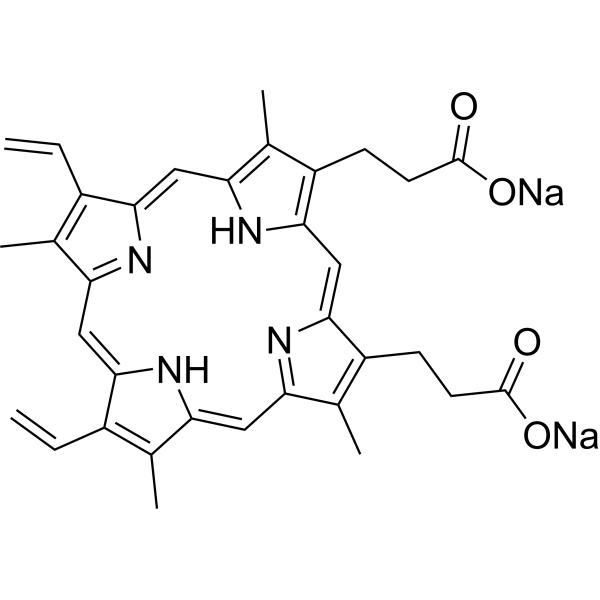
C34H32N4NA2O4
|
|---|---|
| 分子量 |
606.62
|
| 精确质量 |
606.22
|
| 元素分析 |
C, 67.32; H, 5.32; N, 9.24; Na, 7.58; O, 10.55
|
| CAS号 |
50865-01-5
|
| 相关CAS号 |
553-12-8
|
| PubChem CID |
71484
|
| 外观&性状 |
Typically exists as solids at room temperature
|
| 沸点 |
1128.9ºC at 760 mmHg
|
| 闪点 |
636.6ºC
|
| LogP |
1.371
|
| tPSA |
136.56
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
44
|
| 分子复杂度/Complexity |
995
|
| 定义原子立体中心数目 |
0
|
| SMILES |
C=CC1=C(C)C2=NC1=CC3=C(C)C(=C(C=C4C(=C(CCC(=O)[O-])C(=N4)C=C5C(=C(C)C(=C2)N5)CCC(=O)[O-])C)N3)C=C.[Na+].[Na+]
|
| 别名 |
Protoporphyrin IX (disodium); 50865-01-5; protoporphyrin disodium; Protoporphyrin IX disodium salt; Disodium protoporphyrin IX; Palepron; Protoporphyrin, disodium salt; Protoporphyrin disodium [JAN]; Dojin PM;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6485 mL | 8.2424 mL | 16.4848 mL | |
| 5 mM | 0.3297 mL | 1.6485 mL | 3.2970 mL | |
| 10 mM | 0.1648 mL | 0.8242 mL | 1.6485 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。