| 规格 | 价格 | ||
|---|---|---|---|
| 500mg | |||
| 1g | |||
| Other Sizes |
| 靶点 |
Tyk2 JH2 (IC50 = 0.2 nM); JAK1 JH2 (IC50 = 1 nM)
|
|---|---|
| 体外研究 (In Vitro) |
小分子JAK抑制剂已成为治疗自身免疫性疾病的主要治疗进展。发现传统上靶向该激酶家族催化活性位点的异构体选择性JAK抑制剂是一个巨大的挑战。我们实现TYK2高选择性的策略依赖于靶向TYK2假激酶(JH2)结构域。在此,我们报道了后期的优化工作,包括结构导向设计和水置换策略,从而发现了BMS-986165(11)作为高亲和力JH2配体和TYK2的强效变构抑制剂。除了前所未有的JAK同种型和kinome选择性外,11还显示出优异的药代动力学特性,具有最小的分析责任,并在几种自身免疫性疾病的小鼠模型中有效。基于这些发现,11似乎与所有其他报道的JAK抑制剂不同,并已成为临床开发中第一种以假激酶为导向的治疗方法,作为自身免疫性疾病的口服治疗[1]。
药物化合物包括碳、氢和其他元素的稳定重同位素,在药物开发过程中主要作为定量示踪剂。由于氘化可能会影响药物的药代动力学和代谢特性,因此值得关注[1]。氘代化合物的潜在好处:生物体内的半衰期更长。氘代化合物可能能够增加化合物的药代动力学特性或体内半衰期。这可以促进给药并增强化合物的安全性、有效性和耐受性。第二,提高口服生物利用度。大量未代谢的药物能够达到其作用目标,因为氘化物质减少了肝脏和肠壁中不需要的代谢(首过代谢)量。低剂量下更好的耐受性和活性取决于高生物利用度。 (3)增强新陈代谢功能。氘代物质可以增强药物代谢并减少有害或反应性代谢物的产生。 (四)增强药品安全性。氘代化学物质是无害的,可以减轻或消除药物的不良副作用。 (5) 保持治疗效果。根据早期研究,氘化分子应保持与氢类似物相当的生物效力和选择性。 |
| 体内研究 (In Vivo) |
Deucravacitinib (BMS-986165)治疗的 NZB/W 小鼠中狼疮样疾病得到强烈抑制。Deucravacitinib (BMS-986165) 是安全的且总体耐受性良好。活性药物组(75%)和安慰剂组(76%)没有出现严重不良事件,且非严重不良事件发生的频率相似。口服后,Deucravacitinib (BMS-986165)被迅速吸收,表观消除半衰期为 8-15 小时[1]。
在小鼠中,在IL-23驱动的棘皮病皮肤炎症(银屑病样)模型中评估了11/Deucravatinib(BMS-986165),其中向小鼠耳朵重复皮内注射IL-23诱导了由Th17细胞和IL-22介导的深度表皮增生(棘皮病)和炎性细胞浸润,类似于银屑病的潜在机制(图6)。在该模型中,11/Deucravatinib(BMS-986165)剂量依赖性地保护小鼠免受IL-23诱导的棘皮病,每天两次口服15mg/kg剂量的11,持续9天,证明与阳性对照的抗IL-23附件素一样有效(图6a)。30mg/kg每日两次口服剂量在提供保护方面比抗IL-23附件更有效。组织学评估表明,表皮增生和炎性细胞浸润也以剂量依赖的方式受到抑制,每天两次30mg/kg的高剂量比抗IL-23附件素阳性对照更有效地提供保护(图6b)。皮肤活检的定量聚合酶链式反应(PCR)分析显示,11种方法在阻断炎症细胞因子表达方面非常有效,包括IL-17A、IL-21以及IL-12和IL-23的亚基(图6c)。对研究动物的PK测量表明,7.5、15和30 mg/kg每日两次的剂量分别在19、21和24小时内提供了等于或高于100 nM(IFNα诱导的pSTAT1)的体外小鼠全血IC50值的药物水平。除了银屑病的临床前模型外,11也被证明在结肠炎和狼疮的小鼠模型中非常有效。这些结果表明,在严重联合免疫缺陷病(SCID)小鼠的抗CD40诱导的结肠炎模型中,与抗p40单克隆抗体对照组相比,11只每天两次服用50mg/kg,可抑制99%的峰值体重减轻(IL-12驱动)和70%的组织学评分。此外,在使用NZB/W狼疮易感小鼠的三个月狼疮疾病模型中,口服最高30mg/kg每日一次的剂量时,11具有良好的耐受性,在预防肾炎方面非常有效。在后一项研究中,疗效与抑制研究小鼠全血和肾脏中I型IFN依赖性基因表达密切相关,至少与阻断抗IFNαR抗体一样有效[1]。 干扰素α-2a攻击后干扰素反应基因的表达[2] 在早晨服用Deucravatinib(BMS-986165)或安慰剂2小时后,用临床剂量的IFNα-2a进行体内攻击约3小时后,开始诱导IRG,这可以通过诱导寡腺苷酸合成酶样(OASL)基因来证明,这是一种典型的IRG(数据未显示)。在服用安慰剂的志愿者中,IRG诱导很强,大多数基因的诱导倍数超过10倍。在评估的53个靶基因中,有52个被纳入分析,因为发现补体成分1q(C1Q)不会立即被干扰素诱导,而是在26小时的时间点被诱导,因此被认为不太可能是干扰素诱导的基因表达的直接靶点。与安慰剂组相比,预先服用去克拉伐替尼以剂量依赖的方式强烈抑制了聚集中包含的所有52个基因的表达(图3);攻击后26小时,C1Q表达受到抑制。IFNα-2a的暴露量以剂量依赖的方式增加,因此,阻断信号转导可以抑制IFNα-1a的消耗。IFNα-2a的这种反常增加似乎是deucravacitinib的另一种PD测量方法。 |
| 酶活实验 |
使用均相时间分辨荧光(HTRF)测定法测定所有生物化学效价和选择性,其中化合物显示与荧光探针竞争结合人重组JAK1、JAK2、JAK3和TYK2 JH1结构域蛋白以及TYK2和JAK1 JH2蛋白结构域。生成剂量-反应曲线,以确定通过非线性回归分析得出的抑制50%HTRF信号(IC50)所需的浓度。使用稳定整合的STAT依赖性萤光素酶报告基因测定法测定T细胞中的细胞潜能和选择性,使用IFNα-刺激测定TYK2/JAK1依赖性信号传导,使用IL-23刺激测定TYK2/JAK1依存性信号传导。使用GM-CSF刺激在TF-1细胞中测量JAK2依赖性信号传导。生成剂量-反应曲线,以确定通过非线性回归分析得出的抑制50%细胞反应(IC50)所需的浓度。还使用特异性细胞因子刺激在人和小鼠全血中测量了JAK依赖性信号传导的效力和选择性,并通过细胞染色和流式细胞术测量了特异性STAT蛋白的磷酸化。先前已经报道了所有测定的实验细节。对所有在生物测定中具有活性的化合物进行电子过滤,以获得泛测定干扰化合物(PAINS)常见的结构属性,并发现其为阴性[1]。
|
| 细胞实验 |
使用T细胞中稳定整合的STAT依赖性萤光素酶报告子测定法测定细胞效力和选择性,使用IFNα刺激测量TYK2/JAK1依赖性信号传导,使用IL-23刺激测量TYK2/JAK1依存性信号传导。使用GM-CSF刺激在TF-1细胞中测量JAK2依赖性信号传导。生成剂量-反应曲线,以确定通过非线性回归分析得出的抑制50%细胞反应所需的浓度(IC50)。还使用特定的细胞因子刺激在人和小鼠全血中测量了JAK依赖性信号传导的效力和选择性,并通过细胞染色和流式细胞术测量了特定STAT蛋白的磷酸化。所有测定的实验细节之前都有报道。所有在生物测定中具有活性的化合物都经过了电子过滤,以获得泛测定干扰化合物(PAINS)共有的结构属性,结果为阴性[1]。
|
| 动物实验 |
IL-23-Induced Acanthosis in Mice [1]
Acanthosis was induced in 6–8-week-old C57BL/6 female mice (19–20 g average weight, Jackson Laboratories) by intradermal injection of dual chain, recombinant human IL-23 into the right ear. IL-23 injections were administered every other day from day 0 through day 9 of the study. Treatment groups consisted of eight mice per group. Compound 11/Deucravacitinib (BMS-986165) at 7.5, 15, and 30 mg/kg BID in vehicle (EtOH:TPGS:PEG300, 5:5:90) and vehicle alone dosed BID by oral gavage, with the first dose given the evening before the first IL-23 injection. An anti-IL-23 adnectin (3 mg/kg) and PBS control were administered subcutaneously approximately 1 h prior to the first IL-23 injection and then twice a week thereafter. Ear thickness was measured using a Mitutoyo dial caliper and calculated as the percent change in thickness from the baseline measurement taken on day 0 before initial IL-23 injections for each animal. At the end of the study, IL-23-injected ears as well as naïve control ears were collected from four animals per group for histological examination and gene expression analyses. Terminal blood samples collected via the retro-orbital sinus were used for PK determinations. Statistical analyses were performed using Student’s t tests or ANOVA with Dunnett’s post test. At the end of the study, ears were removed and fixed in 10% neutral-buffered formalin for 24–48 h. The fixed ears were then cut longitudinally, and two pieces were parallel embedded to make the paraffin blocks. The paraffin blocks were then sectioned and placed on microscope slides for H&E staining for histological evaluation. Severity of ear inflammation was scored using an objective scoring system based on the following parameters: extent of the lesion, severity of hyperkeratosis, number and size of pustules, height of epidermal hyperplasia (acanthosis, measured in interfollicular epidermis), and the amount of inflammatory infiltrate in the dermis and soft tissue. The latter two parameters, acanthosis and inflammatory infiltrate, were scored independently on a scale from 0 to 4: 0, none; 1, minimal; 2, mild; 3, moderate; 4, marked. The histological changes were blindly evaluated by a pathologist. Statistical analyses was performed using one-way ANOVA with Dunnett’s test for comparison of each treatment versus the vehicle control. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following oral administration, deucravacitinib plasma Cmax and AUC increased proportionally over a dose range from 3 mg to 36 mg (0.5 to 6 times the approved recommended dosage) in healthy subjects. The steady state Cmax and AUC24 of deucravacitinib following administration of 6 mg once daily were 45 ng/mL and 473 ng x hr/mL, respectively. The steady state Cmax and AUC24 of the active deucravacitinib metabolite, BMT-153261, following administration of 6 mg once daily were 5 ng/mL and 95 ng x hr/mL, respectively. The absolute oral bioavailability of deucravacitinib was 99% and the median Tmax ranged from two to three hours. A high-fat, high-calorie meal decreased Cmax and AUC of deucravacitinib by 24% and 11%, respectively, and prolonged Tmax by one hour; however, this has clinically significant effects on drug absorption and exposure. After a single dose of radiolabeled deucravacitinib, approximately 13% and 26% of the dose was recovered as unchanged in urine and feces, respectively. Approximately 6% and 12% of the dose was detected as BMT-153261 in urine and feces, respectively. The volume of distribution of deucravacitinib at steady state is 140 L. The renal clearance of deucravacitinib ranged from 27 to 54 mL/minute. Metabolism / Metabolites Deucravacitinib undergoes N-demethylation mediated by cytochrome P-450 (CYP) 1A2 to form major metabolite BMT-153261, which has a comparable pharmacological activity to the parent drug. However, the circulating exposure of BMT-153261 accounts for approximately 20% of the systemic exposure of the total drug-related components. Deucravacitinib is also metabolized by CYP2B6, CYP2D6, carboxylesterase (CES) 2, and uridine glucuronyl transferase (UGT) 1A9. Biological Half-Life The terminal half-life of deucravacitinib was 10 hours. Triazole 11/Deucravacitinib (BMS-986165) Shows Minimal Profiling Liabilities, Excellent PK Properties, and Is Highly Efficacious in Inflammatory and Autoimmune Disease Models [1] Having demonstrated excellent potency and functional selectivity for inhibition of TYK2-dependent responses, 11/Deucravacitinib (BMS-986165) was further profiled in vitro and showed minimal liabilities and acceptable PK properties for further advancement (Table 7). Incubation in liver microsomes shows excellent stability (T1/2 > 120 min) across multiple species including human, mouse, rat, monkey, dog, and rabbit. Good permeability is also observed in a Caco-2 assay with moderate efflux (apical-to-basal Pc = 73 nm/s; basal to apical Pc = 740 nm/s; efflux ratio ∼10). Assessment of the potential for drug–drug interactions (DDI) indicates a low overall risk, as no significant inhibition of multiple cytochrome P450 (CYP) isozymes (1A2, 2C9, 2C19, 2D6, and 3A4) or induction of CYP3A4 is observed up to the highest concentration tested (40 μM). Testing of 11 in the hERG potassium channel patch clamp assay gives a low percent inhibition (26 ± 11% at 10 μM), suggesting a low potential for QTc prolongation-associated cardiovascular risk. Protein binding is in the moderate range (12–15% free) across species including human, monkey, and mouse. Aqueous solubility of the crystalline free base form of 11 is low at 5.2 μg/mL but was deemed acceptable for advancement into preclinical studies. Good overall PK parameters were observed with 11 in preclinical studies across multiple species (Table 8). Consistent with the observed low rate of metabolism in the microsomal assays (T1/2 > 120 min), 11 affords low to modest in vivo clearance rates in mouse, dog, and monkey of 13.2, 6.8, and 4.8 mL min–1 kg–1, respectively. Low volume of distribution (Vss) is also observed in the 2–3 L/kg range with moderate half-lives of ∼4–5 h across species. When administered orally at 10 mg/kg, 11 is well absorbed with excellent exposures and high bioavailability (%F > 85) in mouse, dog, and monkey. Circulating primary amide metabolite formation from N-dealkylation of the deuteromethyl amide of 11 was near the lower limits of detection (<2 nM) in these studies, consistent with the effective blocking of this metabolic pathway by deuteration as previously reported within the series. Pharmacokinetic in healthy volunteers [2] After administration, Deucravacitinib (BMS-986165) was rapidly absorbed and exhibited an apparent elimination t 1/2 of 7.9–15.0 h following a single dose, and a mean effective t 1/2 of 7.5–13.1 h after multiple dosing (Figure 1, Table 1). The Cmax and AUCINF showed a greater than proportional increase with ascending doses following a single dose of deucravacitinib (up to 10 mg in the SAD cohort) but showed an apparent dose proportionality at doses ≥10 mg. A similar PK profile was seen following multiple doses in the MAD cohort, with dose proportionality at doses ≥4 mg b.i.d. Modest accumulation (1.4–1.9‐fold) was observed after multiple dosing, with steady‐state reached by day 5, the first day on which Cmin samples were collected. Urinary recovery of unchanged deucravacitinib was assessed in the SAD cohort only, and ranged from 10% to 15% across all doses; renal clearance of deucravacitinib ranged from 27.5 to 54.2 ml/min across the dose range. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In the preregistration clinical trials of deucravacitinib that included data on 1519 subjects, only 1.8% of patients had serum ALT or AST elevations above 5 times ULN, none of which were considered likely due to drug induced liver injury, with myositis accounting for many of the elevations, and underlying alcoholic or nonalcoholic fatty liver disease accounting for a few. Elevations of ALT levels above 3 times the ULN arose in 1.1% to 1.3% of recipients of deucravacitinib in a 24 week trial compared to 1.2% of placebo recipients. While there were no instances of reactivation of hepatitis B in patients receiving deucravacitinib, patients with preexisting HBsAg in serum were excluded from enrollment and most treatment courses were limited in duration. Since its approval and more widespread clinical use, there have been no further reports of clinically apparent liver injury attributed to deucravacitinib, but it has been available for a limited time only. Likelihood score: E (suspected but unproven cause of clinically apparent liver injury including reactivation of hepatitis B). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of deucravacitinib during breastfeeding. Because it is more than 80% bound to plasma proteins, the amount in milk is likely to be low. However, it is well absorbed orally. If deucravacitinib is required by the mother of an older infant, it is not a reason to discontinue breastfeeding, but until more data become available, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. ◈ What is deucravacitinib? Deucravacitinib is a medication that has been approved for the treatment of moderate-to-severe plaque psoriasis. MotherToBaby has a fact sheet on psoriasis & psoriatic arthritis at: https://mothertobaby.org/fact-sheets/psoriasis-and-pregnancy/. A brand name for deucravacitinib is SOTYKTU™.Sometimes when people find out they are pregnant, they think about changing how they take their medication, or stopping their medication altogether. However, it is important to talk with your healthcare providers before making any changes to how you take this medication. Your healthcare providers can talk with you about the benefits of treating your condition and the risks of untreated illness during pregnancy. ◈ I take deucravacitinib. Can it make it harder for me to get pregnant? Studies have not been done to see if deucravacitinib can make it harder to get pregnant. ◈ Does taking deucravacitinib increase the chance of miscarriage? Miscarriage is common and can occur in any pregnancy for many different reasons. Studies have not been done to see if deucravacitinib increases the chance for miscarriage. ◈ Does taking deucravacitinib increase the chance of birth defects? Every pregnancy starts out with a 3-5% chance of having a birth defect. This is called the background risk. Human pregnancy studies have not been with deucravacitinib. Experimental animal studies reported by the manufacturer did not find an increased chance of birth defects. ◈ Does taking deucravacitinib in pregnancy increase the chance of other pregnancy related problems? Studies have not been done to see if deucravacitinib increases the chance for pregnancy-related problems such as preterm delivery (birth before week 37) or low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth). ◈ Does taking deucravacitinib in pregnancy affect future behavior or learning for the child? Studies have not been done to see if deucravacitinib can cause behavior or learning issues for the child. ◈ Breastfeeding while taking deucravacitinib: Deucravacitinib has not been studied for use while breastfeeding. Be sure to talk to your healthcare provider about all of your breastfeeding questions. ◈ If a male takes deucravacitinib, could it affect fertility (ability to get partner pregnant) or increase the chance of birth defects in a partner’s pregnancy? Studies have not been done to see if deucravacitinib could affect male fertility or increase the chance of birth defects in a partner’s pregnancy. In general, exposures that fathers or sperm donors have are unlikely to increase the risks to a pregnancy. For more information, please see the MotherToBaby fact sheet Paternal Exposures at https://mothertobaby.org/fact-sheets/paternal-exposures-pregnancy/. Protein Binding Protein binding of deucravacitinib was 82 to 90% and the blood-to-plasma concentration ratio was 1.26. Toxicity Summary No clinical data is available for experience with deucravacitinib overdosage. If overdosage occurs in any patients, it is recommended to contact the Poison Help line for additional management of the condition according to the drug overdosage protocol. The elimination of deucravacitinib by treatment with hemodialysis was small in extent, up to 5.4% of the dosage per dialysis therapy, and therefore, limiting the use of hemodialysis in the treatment of overdosage or intoxication with deucravacitinib. Various studies using rate models have demonstrated no carcinogenicity was observed in male or female rats that administered deucravacitinib orally at doses up to 15 mg/kg/day, which is 51-fold higher than the MRHD as per AUC comparison. In rats of the female gender, deucravacitinib had no effects on parameters of reproduction such as mating, fertility, or early development of the embryo at orally administered doses up to 50 mg/kg/day which is 171-fold higher than the MRHD, according to the comparison of AUC. In the male rat, deucravacitinib had no effects on mating, sperm morphology, fertility, or early embryonic parameters of their offspring at orally administered doses up to 50 mg/kg/day, which is 224 times higher than the MRHD according to a comparison of AUC. In the SAD cohort, AEs were reported in 11 volunteers (36.7%) who received deucravacitinib and four volunteers (40%) who received placebo; the most frequently reported AE by preferred term was headache (deucravacitinib: 5 volunteers, 16.7%; placebo: 2 volunteers, 20.0%). A total of seven (23.3%) gastrointestinal disorder AEs (system order class) were reported in volunteers receiving deucravacitinib versus no volunteers in the placebo group, with the most common being dyspepsia reported in three volunteers (10%). All AEs were mild in severity, with the exception of one event of presyncope of moderate severity in the placebo group. The most common all‐cause AEs in the SAD cohort are summarized in Table 2.[2] In the MAD cohort, all AEs were mild to moderate in severity, and the overall frequency of AEs was similar in the deucravacitinib (37 volunteers, 82%) and placebo (13 volunteers, 87%) groups. For the deucravacitinib and placebo groups, respectively, the most frequently reported AEs by preferred term were headache (11 volunteers, 24.4%; 5 volunteers, 33.3%), rash (9 volunteers, 20%; 2 volunteers, 13.3%), upper respiratory tract infection (8 volunteers, 17.8%;. 3 volunteers, 20.0%), acne (6 volunteers, 13.3%; 0 volunteers), and nausea (6 volunteers, 13.3%; 2 volunteers, 13.3%). The most common all‐cause AEs in the MAD cohort are summarized in Table 2. All AEs resolved except for one case of urticaria of moderate severity, which occurred in the deucravacitinib 6 mg b.i.d. group, and was considered unrelated to study treatment. A total of seven volunteers discontinued due to AEs: six following administration of deucravacitinib (2 mg b.i.d.: 1 volunteer, chest pain; 4 mg b.i.d.: 1 volunteer, rash; 6 mg b.i.d.: 2 volunteers, urticaria; 12 mg b.i.d.: 1 volunteer, tonsillitis; 12 mg q.d.: 1 volunteer, furuncle) and one volunteer in the placebo group (decreased consciousness).[2] Deucravacitinib was associated with an increased incidence of skin rashes and acne‐ and urticaria‐like skin reactions versus placebo, particularly at the highest dosage of 24 mg/day (12 mg b.i.d. dose panel). Skin rash/acne AEs were of mild or moderate severity, responded well to topical treatment (corticosteroid cream for urticaria‐like rash and benzoyl peroxide cream, clindamycin solution, or chlorhexidine ointment for acne) if required, and rarely led to discontinuation.[2] White blood cell counts were monitored by a standard automated cell count and further enumeration of major white blood cell populations using TBNK flow cytometry identified no abnormalities in T, B, or natural killer (NK) cell subsets following exposure to deucravacitinib (data not shown).[2] There were no dose‐related trends in the occurrence of any clinical laboratory marked abnormalities or ECG abnormalities, including out‐of‐range ECG intervals, in any part of the study, and no effect of deucravacitinib on heart rate or body temperature was observed.[2] |
| 参考文献 |
|
| 其他信息 |
Pharmacodynamics
Deucravacitinib is a tyrosine kinase 2 (TYK2) inhibitor that works to suppress the immune signaling pathways in inflammatory disorders, such as plaque psoriasis. In clinical studies comprising patients with psoriasis, deucravacitinib reduced psoriasis-associated gene expression in psoriatic skin in a dose dependent manner, including reductions in IL-23-pathway and type I IFN pathway regulated genes. Following 16 weeks of once-daily treatment, deucravacitinib reduced inflammatory markers such as IL-17A, IL-19 and beta-defensin by 47 to 50%, 72%, and 81 to 84%, respectively. Deucravacitinib does not affect with JAK2-dependent hematopoietic functions. Small molecule JAK inhibitors have emerged as a major therapeutic advancement in treating autoimmune diseases. The discovery of isoform selective JAK inhibitors that traditionally target the catalytically active site of this kinase family has been a formidable challenge. Our strategy to achieve high selectivity for TYK2 relies on targeting the TYK2 pseudokinase (JH2) domain. Herein we report the late stage optimization efforts including a structure-guided design and water displacement strategy that led to the discovery of BMS-986165 (11) as a high affinity JH2 ligand and potent allosteric inhibitor of TYK2. In addition to unprecedented JAK isoform and kinome selectivity, 11 shows excellent pharmacokinetic properties with minimal profiling liabilities and is efficacious in several murine models of autoimmune disease. On the basis of these findings, 11 appears differentiated from all other reported JAK inhibitors and has been advanced as the first pseudokinase-directed therapeutic in clinical development as an oral treatment for autoimmune diseases. [1] This randomized, double-blind, single- and multiple-ascending dose study assessed the pharmacokinetics (PKs), pharmacodynamics, and safety of deucravacitinib (Sotyktu™), a selective and potent small-molecule inhibitor of tyrosine kinase 2, in 100 (75 active, 25 placebo) healthy volunteers (NCT02534636). Deucravacitinib was rapidly absorbed, with a half-life of 8-15 h, and 1.4-1.9-fold accumulation after multiple dosing. Deucravacitinib inhibited interleukin (IL)-12/IL-18-induced interferon (IFN)γ production ex vivo in a dose- and concentration-dependent manner. Following in vivo challenge with IFNα-2a, deucravacitinib demonstrated dose-dependent inhibition of lymphocyte count decreases and expression of 53 IFN-regulated genes. There were no serious adverse events (AEs); the overall frequency of AEs was similar in the deucravacitinib (64%) and placebo (68%) groups. In this first-in-human study, deucravacitinib inhibited IL-12/IL-23 and type I IFN pathways in healthy volunteers, with favorable PK and safety profiles. Deucravacitinib is a promising therapeutic option for immune-mediated diseases, including Crohn's disease, psoriasis, psoriatic arthritis, and systemic lupus erythematosus. [2] |
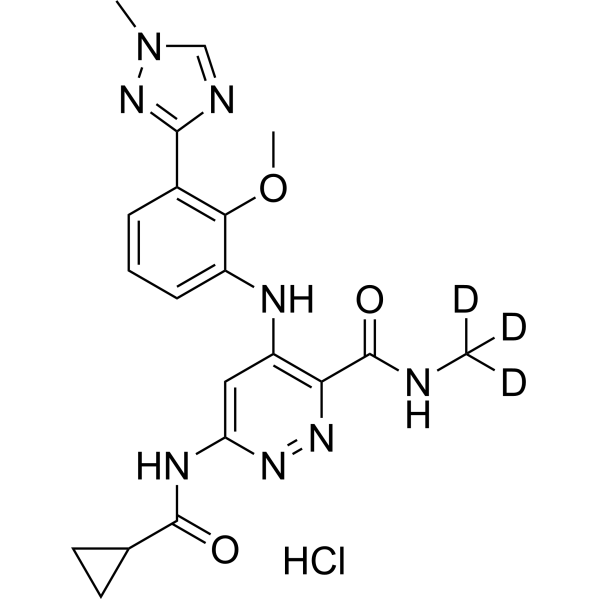
| 分子式 |
C20H23CLN8O3
|
|---|---|
| 分子量 |
461.91990685463
|
| CAS号 |
1609392-28-0
|
| 相关CAS号 |
1609392-27-9
|
| PubChem CID |
146048026
|
| 外观&性状 |
Typically exists as solids at room temperature
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
32
|
| 分子复杂度/Complexity |
648
|
| 定义原子立体中心数目 |
0
|
| SMILES |
Cl.O=C(C1CC1)NC1=CC(=C(C(NC([2H])([2H])[2H])=O)N=N1)NC1C=CC=C(C2N=CN(C)N=2)C=1OC
|
| 别名 |
BMS-986165 hydrochloride; Deucravacitinib hydrochloride; BMS-986165 hydrochloride; 95C5558CF4; UNII-95C5558CF4; 1609392-28-0; 3-Pyridazinecarboxamide, 6-((cyclopropylcarbonyl)amino)-4-((2-methoxy-3-(1-methyl-1H-1,2,4-triazol-3-yl)phenyl)amino)-N-(methyl-d3)-, hydrochloride (1:1)
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1649 mL | 10.8244 mL | 21.6488 mL | |
| 5 mM | 0.4330 mL | 2.1649 mL | 4.3298 mL | |
| 10 mM | 0.2165 mL | 1.0824 mL | 2.1649 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。