| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
Factor Xa
|
|---|---|
| 体外研究 (In Vitro) |
第一种专门针对 Xa 因子的新型抗凝剂是磺达肝素钠。磺达肝素的 IC50 值(抗 Xa IU/ml)对于活化单核细胞 (ac-M) 为 0.59±0.05,对于 MMP(单核细胞衍生微粒)为 0.17±0.03 [2]。
磺达肝癸钠、低分子量肝素和普通肝素对活化单核细胞支持的凝血酶生成的影响[2] 基线时获得的滞后时间值为2.1±0.1 min,峰值值为181±6 nmol/l,ETP值为1624±84 nmol/l min,心率指数值为59±4 nmol/l/min。磺达肝癸钠、依诺肝素和普通肝素以浓度依赖的方式抑制ac-M支持的凝血酶生成(图1)。除滞后时间外,普通肝素的抑制作用优于依诺肝素,依诺肝素在0.3至1.0抗Xa IU/ml之间优于磺达肝素。然而,在最低抗Xa浓度(0.1 IU/ml)下,磺达肝素和依诺肝素对峰值和ETP的抑制作用相当,在0.1 IU/ml抗Xa剂量下,磺达肝素对速率指数的抑制作用比依诺肝素更明显。 IC50的计算[2] 通过计算抑制浓度(IC)50来评估对凝血酶生成的抑制作用,IC 50定义为峰值、ETP和速率指数为产生50%减少的药物浓度,滞后时间为导致其加倍的药物浓度。 在两种模型中,UFH是唯一一种对所有凝血酶生成参数计算IC50的药物。相比之下,在两种模型中,依诺肝素IC50值是针对ETP、峰值和速率指数计算的,而不是针对滞后时间计算的。此外,在两种模型中,仅对速率指数达到磺达肝癸钠的IC50,在MMP模型中仅对峰值和ETP达到IC50。速率指数是三种药物在两种模型中均达到IC50的唯一参数;普通肝素最有效,其次是依诺肝素和磺达肝癸钠(表1)。 |
| 体内研究 (In Vivo) |
磺达肝素钠的药代动力学是线性且剂量依赖性的,从而产生非常可预测的反应。磺达肝素钠起效快,半衰期为 14 至 16 小时,抗血栓活性可持续 24 小时,生物利用度为 100%。该药物对血小板功能或聚集、凝血酶原时间或活化部分凝血活酶时间没有影响[1]。
与急性缺血性综合征组织评估策略(OASIS)-5亚研究中报告的体外抗Xa水平相比,我们的结果表明,患者体内获得的抗Xa值与我们用于达到速率指数IC50的体外模型中所需的值相似,甚至更高磺达肝癸钠在OASIS-5研究中,0.52(±0.22)IU/ml的抗Xa水平足以抑制所测试的两种体外模型中的速率指数。对于依诺肝素,在OASIS-5亚组研究患者中测得的抗Xa水平为1.2(±0.45)IU/ml,明显优于此处测试的两种凝血酶生成模型中抑制速率指数所需的抗-Xa浓度(ac-M IC50:0.27±0.03 IU/ml,MMPs IC50:0.19±0.02 IU/ml) OASIS-5亚研究报告称,与依诺肝素相比,磺达肝癸钠治疗的患者对ETP的抑制作用较小。考虑到OASIS-5亚研究ETP是在无细胞测定中进行的,我们的体外ETP结果与这些数据一致磺达肝癸钠在两种模型中产生的抑制作用均低于依诺肝素,抗Xa浓度范围为0.3至1.0 IU/ml。尽管抗Xa水平升高,但磺达肝藜芦醇的ETP IC50值并不总是达到(磺达肝苷在0.1至1.0抗Xa IU/ml的浓度范围内进行测试),其ETP IC50仅在MMPs模型中达到。相比之下,依诺肝素在这里测试的两种体外模型中达到了ETP IC50。至于速率指数,OASIS-5依诺肝素治疗患者的抗凝水平远优于我们细胞模型中抑制ETP所需的水平。 Oasis-5研究中,每天2.5mg的磺达肝癸钠(相当于0.52±0.22IU/ml的抗Xa)显示出对ACS患者的最佳疗效安全性。磺达肝癸钠的抗Xa水平与ac-M模型的速率指数IC50和MMP模型的ETP IC50值相对应。在这种抗Xa浓度下,MMP模型中的速率指数受到抑制,ac-M模型中未达到ETP IC50。对于依诺肝素,ac-M模型中的速率指数IC50与MMP模型中的ETP IC50相似,接近0.3IU/ml。在这种抗Xa水平下,MMP模型中速率指数受到很大抑制,ac-M中ETP IC50未达到。鉴于此,假设依诺肝素的抗Xa水平约为0.3IU/ml就足以在急性冠状动脉综合征中达到最佳疗效安全性。应该指出的是,在这里分析的三种药物中,磺达肝癸钠是唯一一种合理确定每日剂量的药物,根据之前的剂量发现研究,使用2.5mg剂量就是证明[2]。 |
| 细胞实验 |
单核细胞激活[2]
纯化的单核细胞在RPMI-1640培养基中洗涤,然后在含有5%(v/v)热灭活胎牛血清、2 mmol/l l-谷氨酰胺和100 ng/ml LPS(大肠杆菌血清型O55:B5,Sigma)的RPMI-1640中调节至1.5 106个细胞/ml。单核细胞在37°C、5%CO2加湿的环境中孵育5小时后,通过400g离心5分钟去除上清液。将含ac-M的颗粒重新悬浮在150μl磷酸盐缓冲盐水中。 单核细胞衍生微粒的制备和定量[2] 如上所述,将单核细胞孵育18小时,并通过在2200g下离心5分钟收集上清液。在17000g下额外离心30分钟后回收MMPs,并通过流式细胞术定量[5]。根据制造商的建议,将MMPs(100μl)与1μl膜联蛋白V-FITC在黑暗中孵育15分钟,然后加入400μl膜连蛋白V-结合缓冲液和100μl流计数荧光球。使用System 2软件在Epics XL-MCL流式细胞仪上采集荧光60秒。使用流计数荧光球对MMPs进行定量,并表示为MMPs/μl。 凝血酶生成的荧光测量[2] 凝血酶生成试验根据Hemker描述并经Poitevin修改的测定法进行;ac-Ms和MMPs被定义为细胞模型。用于所有实验条件的PPP系统地补充了终浓度为200激肽释放酶抑制单位(KUI)/ml的抑肽酶。PPP中添加了浓度越来越高的磺达肝癸钠、低分子量肝素或普通肝素;最终浓度为0.0、0.1、0.3、0.6和1.0抗-FXa IU/ml。我们之前优化了检测中包含的ac-Ms和MMPs的浓度。因此,将20μl ac-M(每孔0.2×106ac-M)或MMPs(每孔160000 MMPs)添加到80μl PPP中。结果以百分比表示,基线测量值为100%。 在添加荧光底物Z-Gly-Gly-Arg-AMC后,在Fluoroskan Ascent平板读数器上进行凝血酶生成的荧光测定;用于计算凝血酶生成的凝血酶镜软件。分析了凝血酶生成曲线的四个参数:滞后时间(min)、凝血酶峰值(峰值,nmol/l)、内源性凝血酶电位(ETP,nmol/l-min)、由公式定义的增殖期速率指数:峰值/(达到峰值的时间-滞后时间)(速率指数,nmol/l/min)。 |
| 动物实验 |
Platelet-poor plasma preparation [2]
Venous blood samples were obtained from five healthy volunteers (mean age 25 ± 2 years). Volunteers were laboratory staff members and did not receive any medication for the last 2 weeks. Informed consents were obtained from all of them. Blood was withdrawn by antecubital venipuncture into Monovette tubes (0.106 mol/l citrate). A three-step centrifugation procedure including 10 min at 190g, 10 min at 1750g and 30 min at 13 000g was used. Platelet-poor plasma (PPP) supernatants were pooled, stored at −80°C and thawed immediately before use. Monocyte purification [2] Cytapheresis material was obtained from six healthy volunteers who were admitted for platelet donation in the blood transfusion unit of CHU Robert Debré. Informed consent was obtained from the participants. Monocytes were purified from cytapheresis residues by elutriation, as previously described. Monocyte purity was assessed by CD14 staining of isolated cells (>95% CD14-positive). Cell viability (>98%) was determined by the trypan blue exclusion principle. Purified monocytes were used for preparing ac-Ms and MMPs. |
| 药代性质 (ADME/PK) |
The pharmacology and mechanism of action of fondaparinux sodium are described. Fondaparinux sodium is the first agent of a new class of anticoagulants that selectively target factor Xa. It has a linear, dose-dependent pharmacokinetic profile, which provides a highly predictable response. It is 100% bioavailable, has a rapid onset of action, and has a half-life of 14 to 16 hours, allowing for sustained antithrombotic activity over a 24-hour period. The drug does not affect prothrombin time or activated partial thromboplastin time, nor does it affect platelet function or aggregation. Studies in patients with confirmed heparin-induced thrombocytopenia demonstrate that the drug is not associated with in vitro cross-reactivity to heparin antibodies. Fondaparinux sodium appears to meet the criteria for an ideal antithrombotic agent: equal or better effectiveness than currently available agents, a low bleeding risk, no need for laboratory monitoring, and once-daily administration.[1]
|
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Fondaparinux therapy is associated with a low rate of serum aminotransferase elevations during therapy, with levels above 3 times the upper limit of normal occurring in 1% to 3% of patients, a lower rate than occurs with heparin (~8%) or low molecular weight heparins (4% to 12%) but higher than occurs with placebo ( Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Fondaparinux is considered to be acceptable to use during breastfeeding. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Interactions To investigate the in vitro metabolism of the antithrombotic agent fondaparinux sodium in mammalian liver fractions and to evaluate its potential inhibitory effect on human cytochrome P450 (CYP)-mediated metabolism of other drugs. Metabolism was evaluated by incubating radioisotope-labelled fondaparinux sodium with postmitochondrial liver fractions of rat, rabbit, monkey or human origin (three subjects). Human liver microsomal preparations and an NADPH-generating system were incubated with phenacetin, coumarin, tolbutamide, S-mephenytoin, bufuralol, chlorzoxazone or nifedipine. These are selectively metabolised by CYP isoforms: CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1 or CYP3A4, respectively. Experiments were designed to determine apparent K(i) (inhibitory constant) values for fondaparinux sodium against each CYP isoform, by varying concentrations of fondaparinux sodium and the selective substrate. Each experiment included control reaction mixtures containing an isoform-selective inhibitor. After incubation, the mixtures were analysed by LC-MS/MS or with fluorometric detection. All liver fractions were enzymatically active, as demonstrated by degradation of [(14)C]testosterone. No metabolism of fondaparinux sodium was detectable in postmitochondrial liver fractions. Apparent K(i) values for fondaparinux sodium against the CYP isoforms could not be determined because the oxidative metabolism of the isoform-selective CYP substrates was not significantly inhibited in pooled microsomal reaction mixtures. In the presence of selective CYP inhibitors, metabolism of each substrate was significantly reduced, confirming that inhibition could be observed in these assays. The demonstrated lack of mammalian hepatic metabolism of fondaparinux sodium is consistent with animal and human studies. The absence of inhibition of the human CYP isoforms commonly involved in the metabolism of drugs suggests that clinical treatment with fondaparinux sodium is unlikely to interfere with the pharmacokinetics and metabolism of a wide range of other drugs which are associated with CYP inhibition. PMID:12383041 ... Two separate studies assessed any possible interaction between fondaparinux sodium at steady state and aspirin (acetylsalicylic acid) or piroxicam in healthy volunteers. In the first study a single dose of aspirin 975mg was assessed initially, followed by single doses of aspirin or placebo on the fourth day of an 8-day regimen of subcutaneous fondaparinux sodium (10mg once daily). The second study was a three-way crossover, double-blind, randomised study which investigated fondaparinux sodium 10mg + placebo, fondaparinux sodium 10mg + piroxicam 20mg, or placebo + piroxicam 20mg. ... Neither aspirin nor piroxicam influenced the pharmacokinetics of fondaparinux sodium at steady state. Two hours after administration, prolongation of bleeding time with aspirin alone or with aspirin plus fondaparinux sodium was significantly greater than with fondaparinux sodium alone (p = 0.003 and p = 0.004, respectively). No significant differences were observed between aspirin alone or aspirin + fondaparinux sodium in effect on bleeding time. A small decrease in collagen-induced platelet aggregation was observed after administration of piroxicam alone or piroxicam + fondaparinux sodium. A small effect on aPTT was observed; it was similar for fondaparinux sodium whether administered alone or in combination with either aspirin or piroxicam. No serious adverse events were reported. PMID:12383043 ... In this study /investigators/ assessed the possible pharmacokinetic and pharmacodynamic interaction of fondaparinux sodium with digoxin at steady state in healthy male volunteers. In a phase I randomised, crossover study, volunteers (n = 24) were treated in two periods. The first period was once-daily administration of fondaparinux sodium 10mg subcutaneously alone for 7 days; the second period was 7 days of digoxin 0.25mg orally alone followed by 7 days of coadministration with fondaparinux sodium 10mg. Each period was separated by a washout of 12 days. ... The pharmacokinetic profiles of both digoxin and fondaparinux sodium were unaffected by coadministration. Bioequivalence was concluded, based on the 90% confidence intervals of the ratio of adjusted geometric means calculated for the 2-by-2 comparison of peak concentration, area under the concentration-time curve and cumulative urinary excretion, which lay within the 0.80 to 1.25 reference interval. There were no clinically significant fluctuations in vital signs and ECG parameters. The coadministration of digoxin with fondaparinux sodium was well tolerated and no significant changes were observed in vital signs. Protein Binding 94% in vitro protein binding specifically to ATIII |
| 参考文献 |
|
| 其他信息 |
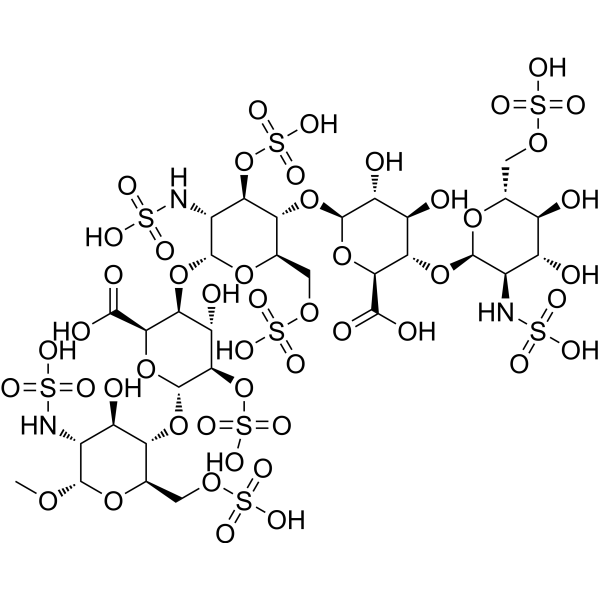
Fondaparinux is a synthetic pentasaccharide which, apart from the O-methyl group at the reducing end of the molecule, consists of monomeric sugar units which are identical to a sequence of five monomeric sugar units that can be isolated after either chemical or enzymatic cleavage of the polymeric glycosaminoglycans heparin and heparan sulfate. It has a role as an anticoagulant. It is an amino sugar, an oligosaccharide sulfate and a pentasaccharide derivative. It is functionally related to a normethylfondaparinux. It is a conjugate acid of a fondaparinux(10-).
Fondaparinux (Arixtra) is a synthetic anticoagulant agent consisting of five monomeric sugar units and a O-methyl group at the reducing end of the molecule. It is structurally similar to polymeric glycosaminoglycan heparin and heparan sulfate (HS) when they are cleaved into monomeric units. The monomeric sequence in heparin and HS is thought to form the high affinity binding site for the natural anti-coagulant factor, antithrombin III (ATIII). Once bound to heparin or HS, the anticoagulant activity of ATIII is potentiated by 1000-fold. Fondaparinux potentiates the neutralizing action of ATIII on activated Factor X 300-fold. Fondaparinux may be used: to prevent venous thromboembolism in patients who have undergone orthopedic surgery of the lower limbs (e.g. hip fracture, hip replacement and knee surgery); to prevent VTE in patients undergoing abdominal surgery who are are at high risk of thromboembolic complications; in the treatment of deep vein thrombosis (DVT) and pumonary embolism (PE); in the management of unstable angina (UA) and non-ST segment elevation myocardial infarction (NSTEMI); and in the management of ST segment elevation myocardial infarction (STEMI). Fondaparinux is a Factor Xa Inhibitor. The mechanism of action of fondaparinux is as a Factor Xa Inhibitor. Fondaparinux is a synthetic inhibitor of factor Xa which given by injection and is used as an anticoagulant to treat as well as prevent venous thromboembolism. Fondaparinux has been associated with a low rate of serum aminotransferase elevations during therapy, but has not been implicated in cases of clinically apparent, idiosyncratic liver injury. Fondaparinux is a synthetic glucopyranoside with antithrombotic activity. Fondaparinux selectively binds to antithrombin III, thereby potentiating the innate neutralization of activated factor X (Factor Xa) by antithrombin. Neutralization of Factor Xa inhibits its activity and interrupts the blood coagulation cascade, thereby preventing thrombin formation and thrombus development. FONDAPARINUX is a Oligosaccharide drug with a maximum clinical trial phase of IV (across all indications) that was first approved in 2001 and has 6 approved and 12 investigational indications. This drug has a black box warning from the FDA. Synthetic pentasaccharide that mediates the interaction of HEPARIN with ANTITHROMBINS and inhibits FACTOR Xa; it is used for prevention of VENOUS THROMBOEMBOLISM after surgery. Fondaparinux Sodium is the sodium salt form of fondaparinux, a synthetic glucopyranoside with antithrombotic activity. Fondaparinux sodium selectively binds to antithrombin III, thereby potentiating the innate neutralization of activated factor X (Factor Xa) by antithrombin. Neutralization of Factor Xa inhibits its activity and interrupts the blood coagulation cascade, thereby preventing thrombin formation and thrombus development. (NCI05) Synthetic pentasaccharide that mediates the interaction of HEPARIN with ANTITHROMBINS and inhibits FACTOR Xa; it is used for prevention of VENOUS THROMBOEMBOLISM after surgery. See also: Fondaparinux (has active moiety). Drug Indication 1. 5-mg/0. 3-ml and 2. 5-mg/0. 5-ml solution for injectionPrevention of venous thromboembolic events (VTE) in adults undergoing major orthopaedic surgery of the lower limbs such as hip fracture, major knee surgery or hip-replacement surgery. Prevention of VTE in adults undergoing abdominal surgery who are judged to be at high risk of thromboembolic complications, such as patients undergoing abdominal cancer surgery. Prevention of VTE in adult medical patients who are judged to be at high risk for VTE and who are immobilised due to acute illness such as cardiac insufficiency and / or acute respiratory disorders, and / or acute infectious or inflammatory disease. Treatment of adults with acute symptomatic spontaneous superficial-vein thrombosis of the lower limbs without concomitant deep-vein thrombosis. 2. 5-mg/0. 5-ml solution for injectionTreatment of unstable angina or non-ST-segment-elevation myocardial infarction (UA/NSTEMI) in adult patients for whom urgent (< 120 mins) invasive management (PCI) is not indicated. infarction (STEMI) in adult patients who are managed with thrombolytics or who initially are to receive no other form of reperfusion therapy. 5-mg/0. 4-ml, 7. 5-mg/0. 6-ml and 10-mg/0. 8-ml solution for injectionTreatment of adults with acute deep-vein thrombosis (DVT) and treatment of acute pulmonary embolism (PE), except in haemodynamically unstable patients or patients who require thrombolysis or pulmonary embolectomy. 1. 5 mg/0. 3 ml and 2. 5 mg/0. 5 ml, solution for injection: , Prevention of Venous Thromboembolic Events (VTE) in patients undergoing major orthopaedic surgery of the lower limbs such as hip fracture, major knee surgery or hip replacement surgery. , Prevention of Venous Thromboembolic Events (VTE) in patients undergoing abdominal surgery who are judged to be at high risk of thromboembolic complications, such as patients undergoing abdominal cancer surgery (see section 5. 1). , Prevention of Venous Thromboembolic Events (VTE) in medical patients who are judged to be at high risk for VTE and who are immobilised due to acute illness such as cardiac insufficiency and/or acute respiratory disorders, and/or acute infectious or inflammatory disease. , , 2. 5 mg/0. 5 ml, solution for injection: , Treatment of unstable angina or non-ST segment elevation myocardial infarction (UA/NSTEMI) in patients for whom urgent (< 120 mins) invasive management (PCI) is not indicated (see sections 4. 4 and 5. 1). , Treatment of ST segment elevation myocardial infarction (STEMI) in patients who are managed with thrombolytics or who initially are to receive no other form of reperfusion therapy. , , 5 mg/0. 4 ml, 7. 5 mg/0. 6 ml and 10 mg/0. 8 ml solution for injection: , Treatment of acute Deep Vein Thrombosis (DVT) and treatment of acute Pulmonary Embolism (PE), except in haemodynamically unstable patients or patients who require thrombolysis or pulmonary embolectomy. Anticoagulants, including unfractionated heparin (UFH), enoxaparin and fondaparinux, are approved drugs in acute coronary syndrome (ACS). Monocytes and monocyte-derived microparticles (MMPs) play an important procoagulant role in ACS by expressing high tissue factor (TF) levels, which in turn triggers thrombin generation. The objective of our study is to compare the in-vitro inhibitory effect of UFH, enoxaparin and fondaparinux in monocytes and MMP models. Human-elutriated monocytes were activated for 5 and 18 h by lipopolysaccharide to obtain activated monocytes (ac-M) or MMPs, respectively. Thrombin generation inhibition was assessed using ac-M or MMPs mixed with platelet-poor plasma containing increased concentrations of anticoagulants. Thrombin generation inhibition was dose-dependent with a differential effect according to the drug: the highest for UFH, the lowest for fondaparinux. Rate index was the most sensitive parameter. For fondaparinux, its IC50 values (anti-Xa IU/ml) were 0.59±0.05 for ac-M and 0.17±0.03 for MMPs. For enoxaparin, rate index IC50 values were 0.27±0.03 for ac-M and 0.19±0.02 for MMPs. Our data support the notion that cell-induced thrombin generation assay may be a reliable alternative to anti-Xa assessment in determining patient anticoagulation level. [2] In conclusion, our study demonstrates a differential inhibitory effect of the three anticoagulants approved in ACS, the evaluation being performed using cell-induced thrombin generation models. Our results support the conclusion reached in OASIS-5 substudy that lower anticoagulation levels should be considered. Our results clearly show the limitation to answer the effect of an anticoagulant molecule using anti-Xa assays. Thrombin generation is an informative and sensitive assay, which remains difficult to standardize, although improvement of standardization is ongoing. Thrombin generation could be used to evaluate the pharmacodynamic effect of new anticoagulant drugs. Furthermore, thrombin generation assay could be a helpful tool to assess more accurately the level to reach in phase I and II studies.[2] |
| 分子式 |
C31H53N3O49S8
|
|---|---|
| 分子量 |
1508.26
|
| 精确质量 |
1506.95133
|
| 元素分析 |
C, 24.69; H, 3.54; N, 2.79; O, 51.98; S, 17.01
|
| CAS号 |
104993-28-4
|
| PubChem CID |
5282448
|
| 外观&性状 |
Typically exists as solids at room temperature
|
| LogP |
0
|
| tPSA |
872.52
|
| 氢键供体(HBD)数目 |
19
|
| 氢键受体(HBA)数目 |
52
|
| 可旋转键数目(RBC) |
30
|
| 重原子数目 |
91
|
| 分子复杂度/Complexity |
3450
|
| 定义原子立体中心数目 |
25
|
| SMILES |
S(N[C@H]1[C@@H](O[C@@H]2[C@H](C(=O)O)O[C@H]([C@@H]([C@H]2O)OS(=O)(=O)O)O[C@@H]2[C@@H](COS(=O)(=O)O)O[C@@H]([C@@H]([C@H]2O)NS(=O)(=O)O)OC)O[C@H](COS(=O)(=O)O)[C@H]([C@@H]1OS(=O)(=O)O)O[C@H]1[C@@H]([C@H]([C@@H]([C@@H](C(=O)O)O1)O[C@@H]1[C@@H]([C@H]([C@@H]([C@@H](COS(=O)(=O)O)O1)O)O)NS(=O)(=O)O)O)O)(=O)(=O)O
|
| 别名 |
Fondaparinux free acid; Fondaparinux; 104993-28-4; Natural heparin pentasaccharide; Arixtra; CHEBI:61033; UNII-J177FOW5JL; J177FOW5JL; HSDB 7845;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.6630 mL | 3.3151 mL | 6.6302 mL | |
| 5 mM | 0.1326 mL | 0.6630 mL | 1.3260 mL | |
| 10 mM | 0.0663 mL | 0.3315 mL | 0.6630 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Study of Arixtra (Fondaparinux Sodium) to Prevent Blood Clots in Women Undergoing Abdominopelvic Surgery for Likely Gynecologic Malignancy
CTID: NCT00539942
Phase: Phase 3 Status: Terminated
Date: 2017-03-27