| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
In a series of metabolism studies, [phenyl-U-14C]-AE 0172747 (/tembotrione/ Batch # Z 31053-4; radiochemical purity 99.5%) or [cyclohexyl-UL-14C]-AE 0172747 (/tembotrione/ Batch #s BECH 1517 or BECH 1523; radiochemical purity >98%) in PEG 200 was administered by oral gavage to groups of four Wistar rats/sex/dose at doses of 5 or 1000 mg/kg. The concentration time-courses of radioactivity in blood and plasma were calculated, the concentrations of radioactivity in tissues and excreta were determined, and metabolites were identified and quantified in the urine and feces. The test compound was absorbed rapidly, as radioactivity was detected in the blood and plasma of all animals at the first time point measured (30 min post-dosing) for both radiolabeled forms. Males had higher mean blood and plasma maximum concentrations (Cmax) than females. Also, males displayed higher AUC values than females in both blood and plasma at both doses. In both sexes, the AUC for both blood and plasma indicated a disproportionally higher mean systemic exposure at 1000 mg/kg than at 5 mg/kg (>200-fold) that was apparently due to a saturation of the initial elimination/biotransformation processes, resulting in a slower initial elimination phase. Other blood and plasma parameters were generally similar across doses and radiolabeled forms. In the 5 mg/kg animals dosed with either radiolabeled form, the liver and kidneys contained the highest mean levels of radioactivity. No other tissue exceeded 0.12% of the administered dose. In the 1000 mg/kg animals dosed with [phenyl-U-14C]-AE 0172747, the skin/fur and carcass contained the highest mean levels of radioactivity. No other tissue exceeded 0.06% of the administered dose. In the 5 mg/kg [phenyl-U-14C] males, the highest concentrations of radioactivity were detected in the, liver, kidneys, skin, and carcass. In the 5 mg/kg [phenyl-U-14C] females and [cyclohexyl- UL-14C] males and females, the highest concentrations of radioactivity were detected in the liver, kidneys, skin, and carcass. In the 1000 mg/kg [phenyl-U-14C] males and females, the highest concentrations of radioactivity were detected in the skin, liver, kidneys, stomach (and contents), and carcass and there was no evidence of bioaccumulation. Total recoveries ranged from 96.3-102.7% of the administered doses, with no differences observed between dose levels or position of the radiolabel. Substantial sex differences were observed in the routes of excretion. At 5 mg/kg, the majority of the radioactivity was recovered in the feces of the males, while in the females, the majority of the radioactivity was recovered in the urine. At this dose, the majority of the radioactivity in the urine was recovered during the first 6 h, while the majority of radioactivity in the feces was recovered during the first 24 h. Tissues and cage wash each accounted for <5.1%. Sex differences in the routes of excretion were also observed in the 1000 mg/kg group. In the males, approximately equal proportions of radioactivity were recovered in the feces and urine, while in the females, the majority of the radioactivity was recovered in the urine. At this dose, the majority of the radioactivity in the urine was recovered during the first 24 h, while the majority of radioactivity in the feces was recovered during the first 48 h. Tissues and cage wash each accounted for <10.1%. The test compound was extensively metabolized. The majority of radioactivity in urine and fecal extract samples was present as parent and up to eleven metabolites. Metabolic profiles were qualitatively similar for both radiolabeled forms; however, profiles for the high and low doses were dissimilar, and major differences were noted between sexes. The major route of metabolism was found to be hydroxylation (oxidative pathway) of the cyclohexyl ring of the molecule. In excreta, parent and identified compounds accounted for 68.1-93.2% of the administered dose, while unidentified metabolites accounted for 2.5-13.8% of the administered dose. The total administered dose accounted for in the excreta was 82.3-104.9%. Parent compound accounted for 1.9-59.9% of the total radioactivity eliminated, and was found in greatest quantity in the urine of the females (44.1-59.4%). Low dose males eliminated small amounts of parent (1.9-3.0%), while high dose males eliminated moderate amounts (33.8%). The metabolite found in the greatest quantity at both doses was 4-hydroxy-AE 0172747, with low dose males eliminating more than low dose females. High dose males and females eliminated approximately equal amounts. The only other metabolite found at >5% of the administered dose was 5-hydroxy-AE 0172747. Males excreted greater quantities than females. Rat metabolism data indicate that tembotrione is well absorbed. More than 96.3% of the administered dose was recovered in urine and feces in 24 hours. Sex differences were observed in the routes of excretion. The primary routes of elimination were the urine in females and the urine and feces in males. At the low dose, males excreted up to 24.4% and 70.4%; females up to 79.1% and 20% of the administered dose in the urine and feces, respectively. At the high dose, females excreted up to 63.7% and 28.5%; males up to 44.2 % and 49.1% of the dose in the urine and feces, respectively. The highest mean levels of radioactivity were extracted from the liver (1.7-3.5%) and kidneys (0.14-0.26%) at the low dose. At the high dose, the mean levels of radioactivity were extracted from the skin/fur (0.22-0.33%) and carcass. The highest concentrations of radioactivity were found in the skin followed by the liver, kidneys, stomach (and contents) and carcass. Males had higher mean blood plasma maximum concentrations (Cmax) and AUC values than females. In both sexes, the area under the AUC for both blood and plasma indicated a disproportionally higher mean systemic exposure at 1000 mg/kg than at 5 mg/kg (>200-fold) that was apparently due to a saturation of the initial elimination/biotransformation processes, resulting in a slower initial elimination phase. In an in vivo dermal penetration study, [phenyl- UL-14C]-AE 0172747 (/tembotrione/ >98% radiochemical purity; batch # BECH 0857) in a suspension concentrate formulation containing 420 g/L AE 0172747 and 210 g/L Isoxadifen-ethyl was applied to four male Wistar (Rj:WI[IOPS HAN]) rats/group on 2 x 6 sq cm skin areas at dose levels of 0, 6.6, 66, or 660 ug/sq cm. Exposure times were 0.5, 1, 2, 4, 10, and 24 hr for each dose. At the end of each exposure period, the skin was swabbed, and urine, feces, treated skin, cardiac blood, kidneys, liver, brain, spleen, and residual carcass were collected and analyzed for radioactivity. Recovery of the applied dose was 90.8-98.7% of the administered dose. The distribution profile of radioactivity was qualitatively similar between the dose groups. The majority of the administered dose was recovered from the skin swabs, accounting for 76-93% of the administered doses. A total of 76-94% of the applied doses was not absorbed. A general trend of increasing dermal absorption with increasing time was observed, and the amount of radioactivity found in the treated skin generally increased with decreasing dose level. Estimates of dermal absorption were based on the sum of the treated skin + the total directly absorbed (urine + feces + cage wash + carcass + brain + spleen + liver + kidneys + blood + non-treated skin + surrounding skin). Dermal absorption was 8.3-14.9% (low), 4.8-12.8% (intermediate), and 1.7- 4.8% (high) of the applied doses. The amount of dermal absorption was not proportional to dose. All treatments (dose levels applied) were for exposure periods for up to 24 hr. The most conservative value for risk assessment is a dermal-absorption of 15% observed at the low dose (6.6 ug/sq cm) at 4 hr after application. This value should be considered to protect commercial applicators. Metabolism / Metabolites The parent molecule and 11 metabolites were identified & isolated from urine and feces /of the rat/. Metabolic profiles were qualitatively similar for both radiolabeled forms; however, profiles for the high and low doses were not the same and differences were noted between sexes. Females excreted the greatest quantity of the parent molecule in urine (44.1-59.4%). While low and high dose males eliminated 1.9-3.0% and 33.8%, respectively, in the urine. The metabolites found in the greatest quantities were 4-hydroxy-tembotrione and 5-hydroxy-tembotrione. Other identified metabolites found at <5% were the 4,5-dihydroxy, benzylic alcohol, dihydroxybezophenone, 4-hydroxy-benzylic alcohol, and ketohydroxy-hexanoic acid ([cyclohexyl-UL-14C] only). Males excreted greater quantities of both major metabolites than females; except, at the high dose where 4-hydroxy-tembotrione was eliminated in approximately equal amounts in both sexes. The primary step in the metabolism of tembotrione is the hydroxylation (oxidative pathway) of the cyclohexyl ring of the molecule. In a series of metabolism studies (MRIDs 46695726, 46695727, 46695728, and 46695729), [phenyl-U-14C]-AE 0172747 (Batch # Z 31053-4; radiochemical purity 99.5%) or [cyclohexyl-UL-14C]-AE 0172747 (Batch #s BECH 1517 or BECH 1523; radiochemical purity >98%) in PEG 200 was administered by oral gavage to groups of four Wistar rats/sex/dose at doses of 5 or 1000 mg/kg. The concentration time-courses of radioactivity in blood and plasma were calculated, the concentrations of radioactivity in tissues and excreta were determined, and metabolites were identified and quantified in the urine and feces. The test compound was absorbed rapidly, as radioactivity was detected in the blood and plasma of all animals at the first time point measured (30 min post-dosing) for both radiolabeled forms. Males had higher mean blood and plasma maximum concentrations (Cmax) than females. Also, males displayed higher AUC values than females in both blood and plasma at both doses. In both sexes, the AUC for both blood and plasma indicated a disproportionally higher mean systemic exposure at 1000 mg/kg than at 5 mg/kg (>200-fold) that was apparently due to a saturation of the initial elimination/biotransformation processes, resulting in a slower initial elimination phase. Other blood and plasma parameters were generally similar across doses and radiolabeled forms. In the 5 mg/kg animals dosed with either radiolabeled form, the liver and kidneys contained the highest mean levels of radioactivity. No other tissue exceeded 0.12% of the administered dose. In the 1000 mg/kg animals dosed with [phenyl-U-14C]-AE 0172747, the skin/fur and carcass contained the highest mean levels of radioactivity. No other tissue exceeded 0.06% of the administered dose. In the 5 mg/kg [phenyl-U-14C] males, the highest concentrations of radioactivity were detected in the, liver, kidneys, skin, and carcass. In the 5 mg/kg [phenyl-U-14C] females and [cyclohexyl- UL-14C] males and females, the highest concentrations of radioactivity were detected in the liver, kidneys, skin, and carcass. In the 1000 mg/kg [phenyl-U-14C] males and females, the highest concentrations of radioactivity were detected in the skin, liver, kidneys, stomach (and contents), and carcass and there was no evidence of bioaccumulation. Total recoveries ranged from 96.3-102.7% of the administered doses, with no differences observed between dose levels or position of the radiolabel. Substantial sex differences were observed in the routes of excretion. At 5 mg/kg, the majority of the radioactivity was recovered in the feces of the males, while in the females, the majority of the radioactivity was recovered in the urine. At this dose, the majority of the radioactivity in the urine was recovered during the first 6 h, while the majority of radioactivity in the feces was recovered during the first 24 h. Tissues and cage wash each accounted for <5.1%. Sex differences in the routes of excretion were also observed in the 1000 mg/kg group. In the males, approximately equal proportions of radioactivity were recovered in the feces and urine, while in the females, the majority of the radioactivity was recovered in the urine. At this dose, the majority of the radioactivity in the urine was recovered during the first 24 h, while the majority of radioactivity in the feces was recovered during the first 48 h. Tissues and cage wash each accounted for <10.1%. The test compound was extensively metabolized. The majority of radioactivity in urine and fecal extract samples was present as parent and up to eleven metabolites. Metabolic profiles were qualitatively similar for both radiolabeled forms; however, profiles for the high and low doses were dissimilar, and major differences were noted between sexes. The major route of metabolism was found to be hydroxylation (oxidative pathway) of the cyclohexyl ring of the molecule. In excreta, parent and identified compounds accounted for 68.1-93.2% of the administered dose, while unidentified metabolites accounted for 2.5-13.8% of the administered dose. The total administered dose accounted for in the excreta was 82.3-104.9%. Parent compound accounted for 1.9-59.9% of the total radioactivity eliminated, and was found in greatest quantity in the urine of the females (44.1-59.4%). Low dose males eliminated small amounts of parent (1.9-3.0%), while high dose males eliminated moderate amounts (33.8%). The metabolite found in the greatest quantity at both doses was 4-hydroxy-AE 0172747, with low dose males eliminating more than low dose females. High dose males and females eliminated approximately equal amounts. The only other metabolite found at >5% of the administered dose was 5-hydroxy-AE 0172747. Males excreted greater quantities than females. Rat metabolism data indicate that tembotrione is well absorbed. More than 96% of the administered dose was recovered in urine and feces in 24 hours. Minor sex differences were observed in the routes of excretion. The primary routes of elimination were the urine in females and the urine and feces in males. The highest concentrations of radioactivity were found in the skin followed by the liver, kidneys, stomach (and contents) and carcass. Males had higher mean blood, plasma maximum concentrations (Cmax) and area under the concentration-time curves (AUC) values than females. The primary step in the metabolism of tembotrione is the hydroxylation (oxidative pathway) of the cyclohexyl ring of the molecule. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
In a subchronic toxicity study, two groups of 10 male and 10 female Wistar rats (Groups 1 and 3) were fed basal diet while two groups of 10 male and 10 female Wistar rats (Groups 2 and 4) were fed diets supplemented with 20,000 ppm (2%) L-tyrosine (Lot/batch No. 078H06822 and 123K0376; purity >99%) for 28 days. (Tyrosine supplementation was approximately three to five times the normal dietary intake.) Rats in Groups 3 and 4 received 10 ug/kg bw/day 2-(2-nitro-4-trifluoromethyl-benzoyl)-1,3- cyclohexanedione (NTBC), an inhibitor of 4-hydroxyphenylpyruvate dioxygenase, daily by gavage. The study was done to determine the effects of increased plasma tyrosine to the eye, kidney, liver, pancreas, and thyroid of rats. One Group 3 female rat died during the study, but its death was unrelated to treatment. No treatment-related effects were noted on body weight, body weight gain, or food consumption. Nine of ten male and 3/10 female rats in Group 4 (2% tyrosine + 10 ug/kg bw/day NTBC) developed white areas on the eye between Days 23-26 on one or more occasions. Following opthalmoscopic examination prior to sacrifice, 9/10 male rats in Group 4 had developed corneal edema and all male and 3/10 female rats had developed 'snow flake' corneal opacities. In addition, three Group 4 male rats had developed congestive iritis. None of the male and female rats in Group 2 (2% tyrosine) or Group 3 (10 ug/kg bw/day NTBC) developed ocular abnormalities. The average plasma tyrosine concentration of Group 3 and Group 4 male and female rats was markedly increased 18-23 fold on the day of sacrifice, while plasma tyrosine was unaffected by treatment in Group 2 rats. Although the liver to body weight ratio of male and female rats in Group 4 was statistically increased, no histological correlates were found. No other treatment-related effects were noted on organ weight. Microscopic treatment-related effects were found in the pancreas, thyroid, and eyes of Group 4 rats. The incidences of focal/multifocal acinar atrophy/ fibrosis and/or acinar degeneration/apoptosis, as well as the incidence of focal/multifocal or diffuse inflammation were increased in the pancreas of Group 4 male and female rats. In the thyroid, an increased incidence of colloid alteration was found in male, but not female rats of Group 4 rats. In the eye, the incidence of unilateral and bilateral keratitis was markedly increased in male rats while minimal keratitis was found in 1/10 Group 4 female rats. No treatment-related effects were noted in male or female Group 2 and Group 3 rats. In a subchronic toxicity study, two groups of five male and five female Wistar rats (Groups 1 and 3) were fed basal diet while two groups of five male and five female Wistar rats (Groups 2 and 4) were fed diets supplemented with 20,000 ppm (2%) L-tyrosine (Lot No. 114K0375, purity 98.9%) for 28 days. (The tyrosine supplementation was approximately three to five times the normal dietary intake.) Rats in Groups 3 and 4 received 10 ug/kg bw/day 2-(2-nitro-4-trifluoromethyl-benzoyl)-1,3-cyclohexanedione (NTBC), an inhibitor of 4-hydroxyphenylpyruvate dioxygenase, daily by gavage. The study was done to determine the effects of increased plasma tyrosine concentration to the eye, kidney, liver, pancreas, and thyroid of rats No toxicologically significant effects on body weight or food intake were noted. All male and 1/5 female rats in Group 4 (2% dietary tyrosine + 10 ug/kg bw/day NTBC by gavage) developed white areas on the eye beginning on Day 24 through the end of the study. In addition, the eyes of 4/5 Group 4 male rats were half-closed beginning on Day 22 through the remainder of the study. The average plasma tyrosine concentration of Group 4 male and female rats increased with time from approximately three to five fold on Day 2 to a 24-fold increase in males and 18-fold increase in females by Day 21. Treatment with 10 ug/kg bw/day NTBC alone had little effect on plasma tyrosine in male and female rats until Day 29/30 when it was increased 3-fold and 5.8- fold in males and females, respectively. After an overnight fast, plasma tyrosine was increased in NTBC-treated rats 18-fold in males and 27-fold in females. Treatment with 2% dietary tyrosine alone induced a < 5-fold increase of plasma tyrosine in male and female rats that decreased with fasting. There were no effects on the absolute or relative liver, brain, kidney, or thyroid weights of tyrosine-, NTBC, or tyrosine/NTBC-treated rats. Macroscopically, minimal to slight bilateral ocular opacity was observed in all male and 1/5 female rats treated with tyrosine/NTBC and microscopically, treatment-related effects were found in the eye, pancreas, and thyroid. Bilateral keratitis was observed in the eyes of all males and one female and diffuse interstitial mixed cell inflammation was noted in the pancreas of two males and one female rat treated with tyrosine/ NTBC. The pancreatic changes were associated with an increased incidence of focal/multifocal acinar degeneration and apoptosis. Minimal to slight thyroid colloid alteration was noted in 3/5 Group 4 male rats. No treatment-related effects, to the eye, pancreas, or thyroid, were noted in rats treated only with tyrosine or NTBC. This study demonstrated a prolonged threshold tyrosine concentration exists in rats, above which macroscopic and/or microscopic effects occur to the eye, pancreas, and thyroid. These effects occurred when rats were fed diets containing three to five times the normal dietary intake of tyrosine while one of the tyrosine catabolizing enzymes was inhibited. Non-Human Toxicity Values LC50 Rat inhalation > 5.03 mg/L/4 hr LD50 Rat dermal >2,000 mg/kg LD50 Rat oral >2,000 mg/kg |
| 其他信息 |
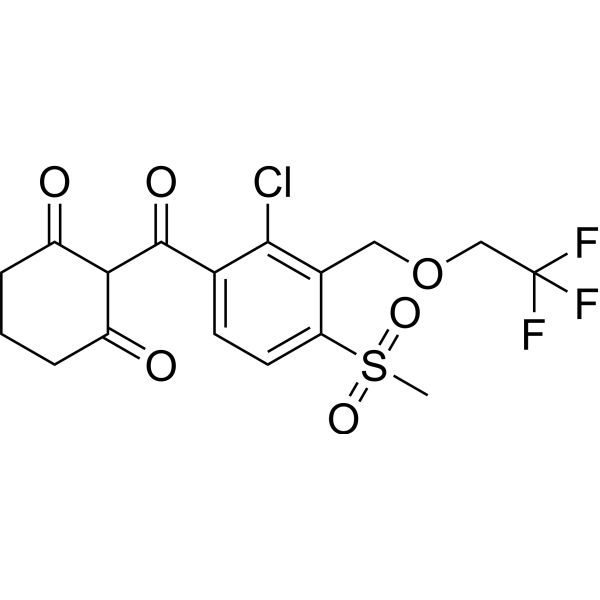
Tembotrione is an aromatic ketone that is 2-benzoylcyclohexane-1,3-dione in which the phenyl group is substituted at positions 2, 3, and 4 by chlorine, (2,2,2-trifluoroethoxy)methyl, and methylsulfonyl groups, respectively. It is a post-emergence herbicide used (particularly in conjunction with the herbicide safener cyprosulfamide) for the control of a wide range of broad-leaved and grassy weeds in corn and other crops. It has a role as a herbicide, an agrochemical, an EC 1.13.11.27 (4-hydroxyphenylpyruvate dioxygenase) inhibitor and a carotenoid biosynthesis inhibitor. It is a sulfone, a cyclic ketone, an aromatic ketone, a member of monochlorobenzenes, an organofluorine compound, an ether and a beta-triketone.
Mechanism of Action Tembotrione is a broad-spectrum early and mid-postemergence herbicide that belongs to the triketone class of herbicides. It acts by inhibiting 4-hydroxyphenylpyruvate dioxygenase (HPPD), which leads to chlorophyll destruction by photooxidation and causes bleaching of emerging foliar tissue. In mammals, HPPD is a key enzyme in the catabolism of tyrosine. It catalyzes the conversion of 4-hydroxyphenylpyruvate (HPP) to homogentisate. Inhibition of HPPD leads to a reconversion of HPP to tyrosine and a consequent increase in blood tyrosine concentrations (tyrosinemia). |
| 分子式 |
C17H16O6F3SCL
|
|---|---|
| 分子量 |
440.81854
|
| 精确质量 |
440.031
|
| CAS号 |
335104-84-2
|
| PubChem CID |
11556911
|
| 外观&性状 |
Beige powder
|
| 密度 |
1.458g/cm3
|
| 沸点 |
612.86ºC at 760 mmHg
|
| 熔点 |
123 °C
MP: 117 °C |
| 闪点 |
324.446ºC
|
| 折射率 |
1.519
|
| LogP |
4.024
|
| tPSA |
102.96
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
9
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
28
|
| 分子复杂度/Complexity |
714
|
| 定义原子立体中心数目 |
0
|
| SMILES |
ClC1C(COCC(F)(F)F)=C(S(C)(=O)=O)C=CC=1C(C1C(=O)CCCC1=O)=O
|
| InChi Key |
IUQAXCIUEPFPSF-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C17H16ClF3O6S/c1-28(25,26)13-6-5-9(15(18)10(13)7-27-8-17(19,20)21)16(24)14-11(22)3-2-4-12(14)23/h5-6,14H,2-4,7-8H2,1H3
|
| 化学名 |
2-[2-chloro-4-methylsulfonyl-3-(2,2,2-trifluoroethoxymethyl)benzoyl]cyclohexane-1,3-dione
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2685 mL | 11.3425 mL | 22.6850 mL | |
| 5 mM | 0.4537 mL | 2.2685 mL | 4.5370 mL | |
| 10 mM | 0.2269 mL | 1.1342 mL | 2.2685 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。