| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
Mycotoxin
|
|---|---|
| 体外研究 (In Vitro) |
霉菌毒素可能存在于作物生长的早期,但直到最近才知道这些真菌代谢实体的真正化学性质。据推测,早在《死海古卷》中报道的时间,就有历史证据表明它们的存在。在20世纪60年代初黄曲霉毒素被发现之前,它们周期性历史发生的证据一直存在。当时,霉菌毒素被认为是一种储存现象,即谷物在储存过程中发霉,从而产生这些次生代谢物,这些代谢物在被人类和其他动物食用时被证明是有毒的。随后,发现在田间作物生长过程中会形成黄曲霉毒素和几种霉菌毒素。确定许多已知的霉菌毒素中哪些是重要的,可以基于它们的发生频率和/或它们产生的疾病的严重程度,特别是如果它们已知是致癌的。属于这一主要类别的霉菌毒素包括黄曲霉毒素、脱氧雪腐镰刀菌烯醇、伏马菌素、Zearalenone/玉米赤霉烯酮、T-2毒素、赭曲霉毒素和某些麦角生物碱。由这些霉菌毒素引起的疾病(霉菌毒素)多种多样,涉及包括人类在内的多种易感动物。这些疾病大多发生在食用受霉菌毒素污染的谷物或由此类谷物制成的产品后,但也存在其他接触途径。由于疾病症状与其他因素引起的症状相似,霉菌中毒的诊断可能很困难。因此,霉菌毒素的诊断取决于对霉菌毒素的充分检测,包括取样、样品制备和分析[2]。
|
| 体内研究 (In Vivo) |
Zearalenone/玉米赤霉烯酮(ZEA)是一种霉菌毒素,主要由食品和饲料中的镰刀菌属真菌产生。它经常与农场动物的生殖障碍有关,偶尔也与人类的高雌激素综合征有关。有证据表明,ZEA及其代谢产物在猪、牛和绵羊体内具有雌激素活性。然而,在小鼠、大鼠和猪口服或腹膜内给药后,ZEA的急性毒性相对较低。ZEA在动物体内的生物转化涉及两种代谢物α-玉米赤霉烯醇(α-ZEA)和β-玉米赤酵母烯醇(β-ZEA)的形成,随后与葡萄糖醛酸结合。此外,ZEA还被证明具有肝毒性、血液毒性、免疫毒性和遗传毒性。ZEA毒性的确切机制尚未完全确定。本文综述了ZEA及其代谢产物的急性、亚急性和慢性毒性、生殖和发育毒性、致癌性、遗传毒性和免疫毒性。ZEA常见于欧洲、非洲、亚洲、美洲和大洋洲的温带地区的几种食物和饲料中。本综述考虑了ZEA在全球范围内对食品和饲料污染的最新数据。由于ZEA造成的经济损失及其对人类和动物健康的影响,文献中描述了几种对受污染食品和饲料进行解毒的策略,包括物理、化学和生物过程。世界上很少有国家报告了ZEA的膳食摄入量。加拿大、丹麦和挪威的ZEA平均膳食摄入量估计为20纳克/千克体重/天,美国为30纳克/千克重量/天。粮农组织/世界卫生组织食品添加剂联合专家委员会(JECFA)为ZEA制定了0.5微克/千克体重的临时最大容许日摄入量(PMDDI)[1]。
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
WHEN (14)C-LABELED ZEARALENONE ADMIN BY GAVAGE TO WHITE LEGHORN LAYING HENS, 94% OF ADMIN (14)C WAS ELIMINATED VIA EXCRETA WITHIN 72 HR. NO MAJOR RETENTION SITES OF (14)C ACTIVITY WERE FOUND, BUT PERSISTENT LEVELS OF LIPOPHILIC METABOLITES WERE DETECTED IN EGG YOLK. ZEARALENONE WITH RADIOACTIVE CARBON WAS ADMIN ORALLY TO RATS & OF PORTION RECOVERED, 70-80% WAS IN FECES & 20-30% IN URINE. CRYSTALLINE ZEARALENONE IN MIXED FEED WAS ADMIN TO MILK COW & EWE. EXTRACTS OF MILK ANALYZED REVEALED TRACES OF ZEARALENONE & BETA-ZEARALENOL IN SOME EXTRACTS OF COW MILK & IN SOME SHEEP MILK. SINGLE EXPOSURE OF LAYING HENS TO FEED CONTAMINATED WITH LOW LEVEL OF ZEARALENONE WOULD PROBABLY RESULT IN MINIMAL HEALTH HAZARD TO HUMANS. HOWEVER, PROLONGED EXPOSURE MIGHT RESULT IN ACCUM OF SIGNIFICANT AMT OF METABOLITE IN EGG YOLK. Metabolism / Metabolites (14)C-LABELED ZEARALENONE ADMIN BY GAVAGE TO WHITE LEGHORN LAYING HENS, ABOUT 94% OF (14)C WAS ELIMINATED VIA EXCRETA WITHIN 72 HR. 1/3 OF DOSE WAS EXCRETED AS UNCHANGED ZEARALENONE, & ANOTHER 1/3 APPEARED AS POLAR METABOLITE. 726 MG OF ZEARALENONE ADMIN IN SINGLE DOSE TO PIG, URINE COLLECTED FOR 96 HR THEREAFTER; 7% OF ZEARALENONE ADMIN WAS RECOVERED IN URINE, 40% OF THIS AS ZEARALENOLS. THE NONSTEROIDAL ESTROGEN ZEARALENONE WAS METABOLIZED BY LIVER HOMOGENATE INTO ALPHA-ZEARALENOL @ PH 4.5 & INTO ALPHA-ZEARALENOL & BETA-ZEARALENOL @ PH 7.4. ZEARALENONE WAS REDUCED TO ZEARALENOL IN FEMALE RAT LIVER BY 3ALPHA-HYDROXYSTEROID DEHYDROGENASE. |
| 毒性/毒理 (Toxicokinetics/TK) |
Zearalenone has been tested for genotoxicity in a variety of test systems; the results were negative, except for the induction of chromosomal aberrations after exposure of mammalian cells in vitro to very high concentrations. Hepatocellular adenomas and pituitary tumours were observed in carcinogenicity studies in mice, but only at doses greatly in excess of the concentrations that have hormonal effects, i.e. ≥ 8-9 mg/kg bw/d. The Committee concluded that these tumours were due to the estrogenic effects of zearalenone and that the safety of zearalenone could be evaluated on the basis of the dose that had no hormonal effect in pigs, the most sensitive species. Using a safety factor of about 100, the Committee established a PMTDI for zearalenone of 0.5 µg/kg bw/d, based on the NOEL of 40 µg/kg bw/d in the 15-day study in pigs. The Committee also considered the LOEL of 200 µg/kg bw per day in this study and the previously established ADI of 0-0.5 µg/kg bw for the metabolite alpha-zearalanol, evaluated as a veterinary drug. The Committee recommended that the total intake of zearalenone and its metabolites (including alpha-zearalanol) should not exceed this value.
Evidence for Carcinogenicity Evaluation: There is inadequate evidence in humans for the carcinogenicity of toxins derived from Fusarium graminearum. No data were available on the carcinogenicity to humans of derived from F. crookwellense and F. culmorum. There is limited evidence in experimental animals for the carcinogenicity of zearalenone. ... Overall evaluation: Toxins derived from Fusarium graminearum, F. culmorum and F. crookwellense are not classifiable as to their carcinogenicity to humans (Group 3). Adverse Effects Dermatotoxin - Skin burns. Toxic Pneumonitis - Inflammation of the lungs induced by inhalation of metal fumes or toxic gases and vapors. 5281576 rat LD50 oral >16 gm/kg Toxicology and Applied Pharmacology., 37(144), 1976 5281576 mouse LD oral >2 gm/kg National Toxicology Program Technical Report Series., NTP-TR-235(1982) 5281576 mouse LD50 intraperitoneal 5 mg/kg Veterinary and Human Toxicology., 25(335), 1983 [PMID:6636506] 5281576 domestic animals - goat/sheep LD50 oral >5 mg/kg Veterinary and Human Toxicology., 25(335), 1983 [PMID:6636506] |
| 参考文献 | |
| 其他信息 |
Zearalenone appears as white microcrystals or white powder. (NTP, 1992)
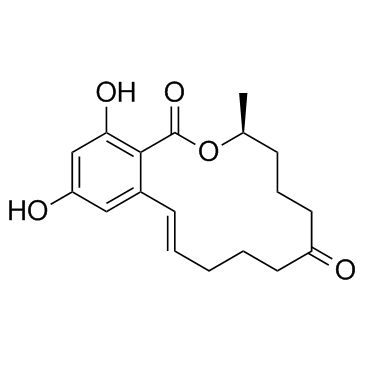
Zearalenone is a macrolide comprising a fourteen-membered lactone fused to 1,3-dihydroxybenzene; a potent estrogenic metabolite produced by some Giberella species. It has a role as a fungal metabolite and a mycoestrogen. It is a macrolide and a member of resorcinols. Zearalenone has been reported in Fusarium graminearum, Fusarium equiseti, and other organisms with data available. (S-(E))-3,4,5,6,8,10-Hexahydro-14,16-dihydroxy-3-methyl-1H-2-benzoxacyclotetradecin-1,7(8H)-dione. One of a group of compounds known under the general designation of resorcylic acid lactones. Cis, trans, dextro and levo forms have been isolated from the fungus Gibberella zeae (formerly Fusarium graminearum). They have estrogenic activity, cause toxicity in livestock as feed contaminant, and have been used as anabolic or estrogen substitutes. |
| 分子式 |
C18H22O5
|
|---|---|
| 分子量 |
318.3643
|
| 精确质量 |
318.146
|
| 元素分析 |
C, 67.91; H, 6.97; O, 25.13
|
| CAS号 |
17924-92-4
|
| PubChem CID |
5281576
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
600.4±55.0 °C at 760 mmHg
|
| 熔点 |
164-165°C
|
| 闪点 |
219.5±25.0 °C
|
| 蒸汽压 |
0.0±1.8 mmHg at 25°C
|
| 折射率 |
1.539
|
| 来源 |
Fusarium graminearum; Fusarium equiseti; Fusarium
|
| LogP |
3.83
|
| tPSA |
83.83
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
0
|
| 重原子数目 |
23
|
| 分子复杂度/Complexity |
445
|
| 定义原子立体中心数目 |
1
|
| SMILES |
C[C@H]1CCCC(=O)CCC/C=C/C2=C(C(=CC(=C2)O)O)C(=O)O1
|
| InChi Key |
MBMQEIFVQACCCH-QBODLPLBSA-N
|
| InChi Code |
InChI=1S/C18H22O5/c1-12-6-5-9-14(19)8-4-2-3-7-13-10-15(20)11-16(21)17(13)18(22)23-12/h3,7,10-12,20-21H,2,4-6,8-9H2,1H3/b7-3+/t12-/m0/s1
|
| 化学名 |
(3S,11E)-14,16-dihydroxy-3-methyl-3,4,5,6,9,10-hexahydro-1H-2-benzoxacyclotetradecine-1,7(8H)-dione
|
| 别名 |
ZEA; RAL; F-2 toxin; F 2 toxin; ZEARALENONE; 17924-92-4; (-)-Zearalenone; trans-Zearalenone; Zenone; (S)-Zearalenone; F2 toxin; Mycotoxin F2; Toxin F2
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~314.11 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1411 mL | 15.7055 mL | 31.4110 mL | |
| 5 mM | 0.6282 mL | 3.1411 mL | 6.2822 mL | |
| 10 mM | 0.3141 mL | 1.5705 mL | 3.1411 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04152265 | UNKNOWN STATUS | Procedure:colonoscopy Other:questionnaires Other:demographic data collections |
Colorectal Cancer,Somatic(Diagnosis) | University of Warmia and Mazury in Olsztyn | 2019-11-01 | Not Applicable |
| NCT01824940 | COMPLETED | Behavioral:Standard care Other:WASH Dietary Supplement:Infant and young child feeding Other:WASH and Nutrition |
Anemia Growth;Stunting,Nutritional |
Johns Hopkins Bloomberg School of Public Health |
2012-11 | Not Applicable |