| 规格 | 价格 | ||
|---|---|---|---|
| 500mg | |||
| 1g | |||
| Other Sizes |
| 靶点 |
TLR4
|
|---|---|
| 体外研究 (In Vitro) |
当配体与Toll样受体4(TLR4)和髓样分化2(MD-2)受体复合物结合时,两条主要的信号通路被激活,涉及不同的衔接蛋白。一种途径依赖于髓样分化标志物88(MyD88),其引发促炎反应,而另一种途径则依赖于含有Toll-IL-1受体(TIR)结构域的衔接物诱导干扰素-β(TRIF),其诱导I型干扰素的产生。在这里,我们发现TLR4激动剂和疫苗佐剂CRX-547是氨基烷基葡糖胺4-磷酸(AGP)类合成脂质a模拟物的成员,在人类细胞中显示出TRIF选择性信号传导,这取决于对大多数脂质a类型上与1-磷酸基团相对应的羧基生物异构体的轻微结构修饰。CRX-547很少或根本不刺激MyD88依赖性信号分子或细胞因子的激活,而其激活TRIF依赖性途径的能力与结构相关的炎性AGP和明尼苏达沙门氏菌脂多糖的能力相似。这种TRIF选择性信号传导反应导致与MyD88信号传导相关的促炎介质的产生大大减少,从而可能降低毒性并提高这种合成TLR4激动剂和疫苗佐剂的治疗指数[1]。
|
| 细胞实验 |
为了进行药理学分析,用各种浓度的CRX-527或CRX-547处理人PBMC衍生的单核细胞和树突状细胞,并通过XLFit软件(IDBS)用四参数逻辑方程拟合得到的剂量反应曲线,以确定ED50(中位有效剂量或半最大有效剂量)值和反应程度。对于抑制实验,用恒定浓度的CRX-547预处理细胞,然后用大范围的CRX-527预处理。为了进行Schild回归,我们通过将曲线的ED50除以仅CRX-527反应曲线的ED50来确定每条抑制曲线的等效剂量比(DR)。对数(DR-1)与对数[CRX-547]的回归用于拟合一条直线,其截距表示CRX-547的Kb估计值。[1]
NF-κB核转位分析[1] 在THP-1培养基中呈指数增长的MonoMac6细胞在指定时间内用越来越高浓度的CRX-527或CRX-547刺激。将细胞在2%的副甲醛中固定过夜,用渗透缓冲液(PBS、2%FBS和0.1%Triton X)处理,并与以下一抗和二抗一起孵育:抗NF-κB抗体(p65)、抗IRF3抗体、异硫氰酸荧光素(FITC)偶联的抗兔抗体抗体和藻红蛋白(PE)偶联的小鼠抗体。如前所述(图4A),信号蛋白定位与核染色剂DRAQ5的相似性得分用于IDEAS软件对核转位的ImageStream分析。简而言之,相似性得分是通过计算核(DRAQ5)的像素强度与染色细胞的NF-κB图像之间的相关性来计算的,这些染色细胞被流体动力学聚焦、激光激发并在电荷耦合器件(CCD)相机上成像。相似性得分是Pearson相关系数的导数,随着NF-κB和核染色的像素强度共同定位,相似性得分达到更高的正值。针对每种测试条件和时间点,收集并分析了至少3000至5000个细胞。 蛋白质印迹分析[1] 对于总蛋白和磷酸化蛋白的蛋白质印迹分析,通过在37°C和5%CO2中粘附6孔培养板2小时,从PBMCs(1×107)中分离出原代人单核细胞,并用CRX-527或CRX-547(各0.1μM)处理指定时间。处理后,用含有蛋白酶抑制剂鸡尾酒的细胞裂解缓冲液裂解细胞。蛋白质通过SDS-聚丙烯酰胺凝胶电泳(SDS-PAGE)分离,并转移到聚偏二氟乙烯膜上。将膜与抗β-肌动蛋白的第一单克隆抗体、抗pIRF3抗体(Ser396)、抗IRAK1的第一多克隆抗体或抗IRF3抗体一起孵育,然后与辣根过氧化物酶(HRP)偶联的抗兔或小鼠抗体的第二抗体(KPL)一起孵育。使用ECL Advance Western Blot试剂盒检测条带。 |
| 参考文献 |
[1]. Selective TRIF-dependent signaling by a synthetic toll-like receptor 4 agonist. Sci Signal. 2012 Feb 14;5(211):ra13.
|
| 其他信息 |
A similar selectivity for TRIF-dependent signaling in mice was reported for MLA. MLA lacks a phosphate on the reducing sugar at a similar position relative to the bioisosteric carboxyl of CRX-527 and CRX-547. The authors of these studies postulated that differences in MyD88- and TRIF-dependent gene expression downstream of TLR4 not only are related to differential activation of NF-κB and IRF3 but also may involve the differential activation of either phosphoinositide 3-kinase or p38 mitogen-activated protein kinase pathways. A recent study by Embry et al. suggests that a defect in IL-1β maturation after stimulation of TLR4 by sMLA is, in part, responsible for the reduced toxicity of MLA compared to that of LPS. Cells activated with sMLA are deficient in inducing the NLRP3 inflammasome (which is MyD88-dependent), as well as that of subsequent inflammasome assembly, caspase-1 activation, and release of mature IL-1β release. Comparison of the effects of synthetic sMLA with those of CRX-547 on human monocytes indicated that CRX-547 was TRIF-selective throughout the range of concentrations tested, whereas sMLA was TRIF-selective only at lower concentrations. Previous studies describing TRIF-dependent signaling by MLA have been conducted in murine systems. These data suggest that important signaling differences may exist for MLA and CRX-547 in human versus murine cells. Overall, our preliminary evaluation of mature IL-1β production by human PBMCs indicated that CRX-547 induced substantially less IL-1β than did CRX-527 (fig. S2), which is consistent with the inefficient activation by CRX-547 of the MyD88-dependent events that are needed for the inflammasome to function. [1]
The selective induction of TRIF-dependent signaling by synthetic lipid A mimetics suggests that further structure-activity investigations of these TLR4 agonists may provide opportunities for a better understanding of the underlying mechanisms that regulate TLR4 signaling pathways. The ability to selectively target either MyD88- or TRIF-dependent signaling downstream of TLR4 with synthetic receptor agonists may also provide valuable insights into the mechanism by which different lipid A structures differentially induce innate and adaptive immune responses. Such studies may be helpful in the development of synthetic vaccine adjuvants or new immunomodulators that selectively alter innate immune responses while simultaneously mitigating the potentially toxic side effects that are associated with the induction of inflammatory cytokines and chemokines. [1] |
| 分子式 |
C81H151N2O19P
|
|---|---|
| 分子量 |
1488.068
|
| 精确质量 |
1487.0649
|
| 元素分析 |
C, 65.38; H, 10.23; N, 1.88; O, 20.43; P, 2.08
|
| CAS号 |
216014-05-0
|
| 相关CAS号 |
216014-14-1 (CRX-527);216014-05-0 (CRX-547);
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| SMILES |
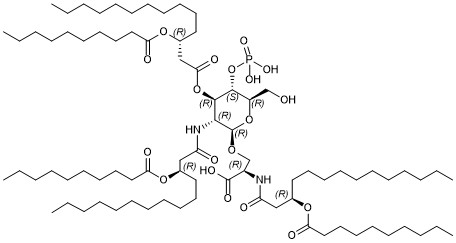
O(C(C[C@H](OC(CCCCCCCCC)=O)CCCCCCCCCCC)=O)[C@@H]1[C@@H](NC(C[C@H](OC(CCCCCCCCC)=O)CCCCCCCCCCC)=O)[C@H](OC[C@@H](NC(C[C@H](OC(CCCCCCCCC)=O)CCCCCCCCCCC)=O)C(O)=O)O[C@H](CO)[C@H]1OP(=O)(O)O
|
| InChi Key |
REEGNIYAMZUTIO-ARLDOHDRSA-N
|
| InChi Code |
InChI=1S/C81H151N2O19P/c1-7-13-19-25-31-34-40-43-49-55-66(97-73(87)58-52-46-37-28-22-16-10-4)61-71(85)82-69(80(91)92)65-96-81-77(83-72(86)62-67(56-50-44-41-35-32-26-20-14-8-2)98-74(88)59-53-47-38-29-23-17-11-5)79(78(70(64-84)100-81)102-103(93,94)95)101-76(90)63-68(57-51-45-42-36-33-27-21-15-9-3)99-75(89)60-54-48-39-30-24-18-12-6/h66-70,77-79,81,84H,7-65H2,1-6H3,(H,82,85)(H,83,86)(H,91,92)(H2,93,94,95)/t66-,67-,68-,69-,70-,77-,78-,79-,81-/m1/s1
|
| 化学名 |
Decanoic acid, (1R)-1-[2-[[(1R)-1-carboxy-2-[[2-deoxy-3-O-[(3R)-1-oxo-3-[(1-oxodecyl)oxy]tetradecyl]-2-[[(3R)-1-oxo-3-[(1-oxodecyl)oxy]tetradecyl]amino]-4-O-phosphono-β-D-glucopyranosyl]oxy]ethyl]amino]-2-oxoethyl]dodecyl ester (9CI, ACI)
|
| 别名 |
CRX-547; CRX547; 216014-05-0; N-[(3R)-3-(Decanoyloxy)myristoyl]-O-[2-[[(3R)-3-(decanoyloxy)myristoyl]amino]-3-O-[(3R)-3-(decanoyloxy)myristoyl]-4-O-phosphono-2-deoxy-beta-D-glucopyranosyl]-D-serine
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.6720 mL | 3.3601 mL | 6.7201 mL | |
| 5 mM | 0.1344 mL | 0.6720 mL | 1.3440 mL | |
| 10 mM | 0.0672 mL | 0.3360 mL | 0.6720 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。