| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
Natural product
|
|---|---|
| 体外研究 (In Vitro) |
从土壤真菌Hamigera avellana BCC 17816中分离出两种新型环丙基二酮hamavellone A(1)和B(2),两种新型14元非酮大内酯hamigeromycin A(3)和B。通过NMR和MS数据分析确定了新化合物的结构。3的绝对立体化学是通过与5的化学相关性来解决的。Hamavellone B(2)表现出抗疟疾活性,IC50为5.2μg/mL,同时也表现出相当的细胞毒性[1]。
|
| 细胞实验 |
使用微培养放射性同位素技术对恶性疟原虫(K1,耐多药菌株)的活性进行测定。17使用绿色荧光蛋白微板测定(GFPMA)对结核分枝杆菌H37Ra的生长抑制活性和对Vero细胞(非洲绿猴肾成纤维细胞)的细胞毒性进行测定。18使用雷沙苏林微板测定评估对白色念珠菌的抗真菌活性和对KB细胞(口腔人类表皮样癌)、MCF7细胞(人类癌症)和NCI-H187细胞(人类小细胞肺癌)的抗癌活性[1]。
|
| 参考文献 | |
| 其他信息 |
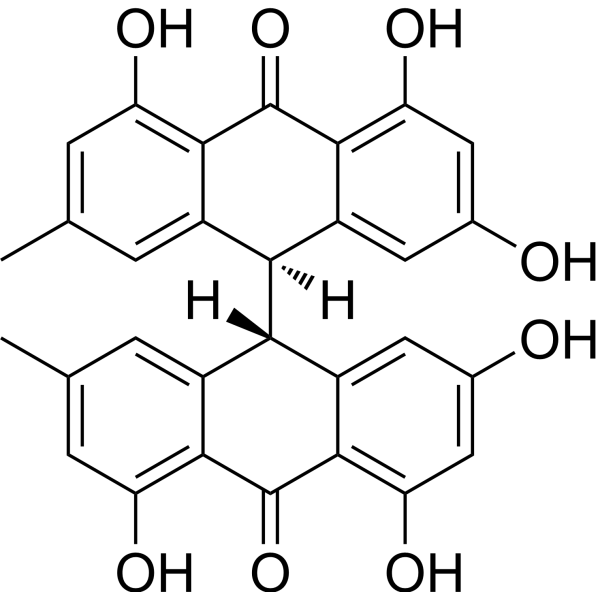
(10S)-1,3,8-trihydroxy-6-methyl-10-[(9S)-2,4,5-trihydroxy-7-methyl-10-oxo-9H-anthracen-9-yl]-10H-anthracen-9-one has been reported in Rhamnus prinoides with data available.
Pseurotin A (6) was first described from Pseudeurotium ovalis S2269/F,3a and it has been isolated from various fungi such as Aspergillus species4, 12 and Pochonia chlamydosporia var. catenulata.13 Emodin (7) and its hydroxy analogue 8 are commonly occurring as constituents of plants and fungi. Emodin bianthrones (9 and 10) and structurally related bianthrones have been known as constituents of plants.5, 6, 6(a), 6(b), 6(c) To the best of our knowledge, this is the first report of the isolation of 9 and 10 as fungal secondary metabolites. There have been only a few other reports on the new metabolites of Hamigera species, such as the cyclopentapeptides, avellanins A and B from H. avellanea14 and their analogues PF1171A–PF1171E from H. avellanea PF1171,15 and polyketide metabolites, hamigerone and dihydrohamigerone from H. avellanea NN005492.16, 16(a), 16(b) In the present study four different chemical classes of polyketide metabolites, comprising 10 compounds, have been isolated from H. avellanea BCC 17816, demonstrating that fungi in this genus are potent sources of bioactive compounds. All isolated compounds were subjected to our in vitro biological protocols, inclusive of antimalarial (Plasmodium falciparum K1), antitubercular (Mycobacterium tuberculosis H37Ra), and antifungal (Candida albicans) activities, and cytotoxicity against three human cancer cell-lines (KB, MCF7, and NCI-H187 cells) and noncancerous Vero cells (Table 3). Hamavellone B (2) exhibited moderate antimalarial activity, while it also showed cytotoxic activities. Among 14-membered macrolides, only compound 5 exhibited moderate cytotoxicity, and no other biological activities were shown in this class of compounds. It should be noted that emodin bianthrones (9 and 10) strongly inhibited the proliferation of the malarial parasite, although they showed comparable cytotoxicity to Vero cells.[1] |
| 分子式 |
C30H22O8
|
|---|---|
| 分子量 |
510.49
|
| CAS号 |
61281-20-7
|
| PubChem CID |
12096290
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.585±0.06 g/cm3(Predicted)
|
| 沸点 |
773.7±60.0 °C(Predicted)
|
| LogP |
5.8
|
| tPSA |
156 Ų
|
| 氢键供体(HBD)数目 |
6
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
38
|
| 分子复杂度/Complexity |
865
|
| 定义原子立体中心数目 |
2
|
| SMILES |
C1(C2(c3c([H])c(O[H])c([H])c(O[H])c3C(=O)c3c(c(c(c([H])c23)C([H])([H])[H])[H])O[H])[H])(c2c([H])c(O[H])c([H])c(O[H])c2C(=O)c2c(c(c(c([H])c12)C([H])([H])[H])[H])O[H])[H]
|
| InChi Key |
UUXPVUHOWQPCSC-ZEQRLZLVSA-N
|
| InChi Code |
InChI=1S/C30H22O8/c1-11-3-15-23(17-7-13(31)9-21(35)27(17)29(37)25(15)19(33)5-11)24-16-4-12(2)6-20(34)26(16)30(38)28-18(24)8-14(32)10-22(28)36/h3-10,23-24,31-36H,1-2H3/t23-,24-/m0/s1
|
| 化学名 |
(10S)-1,3,8-trihydroxy-6-methyl-10-[(9S)-2,4,5-trihydroxy-7-methyl-10-oxo-9H-anthracen-9-yl]-10H-anthracen-9-one
|
| 别名 |
Trans-Emodin bianthrone; ( inverted exclamation markA)-Emodin bianthrone; CHEMBL464711;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9589 mL | 9.7945 mL | 19.5890 mL | |
| 5 mM | 0.3918 mL | 1.9589 mL | 3.9178 mL | |
| 10 mM | 0.1959 mL | 0.9795 mL | 1.9589 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。