| 规格 | 价格 | ||
|---|---|---|---|
| 500mg | |||
| 1g | |||
| Other Sizes |
| 体外研究 (In Vitro) |
目的:免疫抑制剂霉酚酸酯(MMF)诱导的核苷酸耗竭已被证明具有神经保护作用。目前尚不清楚核苷酸耗竭是直接抵消神经元死亡,还是抑制小胶质细胞或星形胶质细胞的激活,从而产生间接的神经保护作用。
方法:通过免疫细胞化学、定量形态计量学和elisa分析MMF对分离的小胶质细胞、星形胶质细胞/小胶质细胞共培养物和分离的海马神经元的影响。 结果:我们发现:(i)MMF抑制脂多糖诱导的小胶质细胞分泌白细胞介素-1β、肿瘤坏死因子-α和一氧化氮;(ii)MMF抑制脂多糖诱导的星形胶质细胞产生肿瘤坏死因子-α,但不抑制一氧化氮;(iii)MMF强烈抑制小胶质细胞和星形胶质细胞的增殖;(iv)MMF不能保护分离的海马神经元免受兴奋性毒性损伤;(v)鸟苷处理后,MMF对神经胶质细胞的作用被逆转。 结论:MMF诱导的核苷酸耗竭抑制了小胶质细胞和星形胶质细胞的活化。MMF诱导的补救途径酶肌苷单磷酸脱氢酶的抑制抑制了小胶质细胞和星形胶质细胞的增殖。先前观察到的MMF治疗后的神经保护作用似乎是间接介导的,使该化合物成为治疗急性中枢神经系统病变的一种有趣的免疫抑制剂[2]。 |
|---|---|
| 体内研究 (In Vivo) |
背景:T淋巴细胞诱导成纤维细胞转化为肌成纤维细胞,这是纤维形成的主要介质。肌苷5'-单磷酸脱氢酶抑制剂霉酚酸酯(MMF)和抗CD25单克隆抗体达利珠单抗(DCZ)已被报道可抑制T淋巴细胞的增殖。
目的:评价MMF和DCZ对博莱霉素(BLM)诱导的硬皮病早期的预防作用。 方法:本研究涉及五组Balb/c小鼠(每组n=10)。其中四组小鼠皮下注射BLM[100μg/天,溶于100μL磷酸盐缓冲盐水(PBS)]4周;其余(对照组)仅接受100μL PBS。三个BLM治疗组也接受了腹腔注射MMF50或150mg/kg/天,或SC DCZ 100μg/周。在第四周结束时,杀死所有小鼠,并采集血液和组织样本进行进一步分析。 结果:在BLM治疗组中,炎症细胞浸润、α-平滑肌肌动蛋白阳性(α-SMA+)成纤维细胞计数、组织羟脯氨酸含量和皮肤厚度均有所增加。皮肤纤维化在组织病理学上表现突出。在BLM治疗的小鼠中,也给予MMF或DCZ,炎症细胞浸润、组织羟脯氨酸含量和皮肤厚度降低。在MMF组中,α-SMA+成纤维细胞计数也有所下降。 结论:在BLM诱导的皮肤纤维化模型中,MMF和DCZ治疗可预防皮肤纤维化的发展。需要进一步的研究来评估靶向T淋巴细胞是否能有效解决人类硬皮病中预先存在的纤维化。[3] |
| 细胞实验 |
小胶质细胞和星形胶质细胞凋亡和增殖分析[2]
用Mycophenolate Mofetil/MMF处理或在培养基中孵育的小胶质细胞或星形胶质细胞用于确定Mycophenolate Mofetil/MMF可能的毒性浓度范围。通过活化的半胱氨酸天冬氨酸蛋白酶-3的免疫细胞化学显示凋亡细胞。在星形胶质细胞/小胶质细胞共培养中对小胶质细胞进行增殖研究,因为只有在星形胶质胶质细胞存在的情况下才能获得显著的小胶质细胞增殖。单独用LPS或巨噬细胞集落刺激因子刺激分离的小胶质细胞没有诱导显著的增殖活性(数据未显示)。分析小胶质细胞增殖的溴脱氧尿苷(BrdU;0.01 mM)加入培养基中16 h固定前。 胎牛血清(1-10%)或促肾上腺皮质激素释放因子(CRF,10 µM)用于48 h用于刺激分离的星形胶质细胞培养物中的增殖,并加入BrdU(0.01 mM)16 h固定前。增殖指数计算为增殖细胞占细胞总数的百分比。 |
| 动物实验 |
Animals and experimental protocols [3]
Fifty specific‐pathogen‐free female Balb/c mice, 6 weeks old and weighing 20–25 g, were used for the experimental procedures. Defined areas of the lower back skins of the mice were shaved for subcutaneous injections. Mice in the control group received 100 μL/day phosphate‐buffered saline (PBS) subcutaneously (SC) to the shaved back skin. To induce dermal fibrosis, the remaining four groups received BLM 100 μg dissolved in 100 μL PBS and sterilized by filtration (0.2 μm filter) to the shaved skin on the back. Two groups of these BLM‐treated mice were also injected either intraperitoneally with MMF 50 or 150 mg/kg/day dissolved in 100 μL and 300 μL saline containing 0.4% Tween 80 and 0.9% benzyl alcohol, respectively, and a third BLM‐treated group was given DCZ 100 μg (100 μL) SC once weekly. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Mycophenolate mofetil is rapidly absorbed in the small intestine. The maximum concentration of its active metabolite, MPA, is attained 60 to 90 minutes following an oral dose. The average bioavailability of orally administered mycophenolate mofetil in a pharmacokinetic study of 12 healthy patients was 94%. In healthy volunteers, the Cmax of mycophenolate mofetil was 24.5 (±9.5)μg/mL. In renal transplant patients 5 days post-transplant, Cmax was 12.0 (±3.82) μg/mL, increasing to 24.1 (±12.1)μg/mL 3 months after transplantation. AUC values were 63.9 (±16.2) μg•h/mL in healthy volunteers after one dose, and 40.8 (±11.4) μg•h/mL, and 65.3 (±35.4)μg•h/mL 5 days and 3 months after a renal transplant, respectively. The absorption of mycophenolate mofetil is not affected by food. A small amount of drug is excreted as MPA in the urine (less than 1%). When mycophenolate mofetil was given orally in a pharmacokinetic study, it was found to be 93% excreted in urine and 6% excreted in feces. Approximately 87% of the entire administered dose is found to be excreted in the urine as MPAG, an inactive metabolite. The volume of distribution of mycophenolate mofetil is 3.6 (±1.5) to 4.0 (±1.2) L/kg. Plasma clearance of mycophenolate mofetil is 193 mL/min after an oral dose and 177 (±31) mL/min after an intravenous dose. /Absorption/ is rapid and extensive after oral administration. In 12 healthy volunteers, the mean absolute bioavailability of oral mycophenolate mofetil relative to intravenous mycophenolate mofetil (based on MPA AUC) was 94%. The area under the plasma-concentration time curve (AUC) for MPA appears to increase in a dose-proportional fashion in renal transplant patients receiving multiple doses of mycophenolate mofetil up to a daily dose of 3 g. Protein binding: To plasma albumin: High (97% for mycophenolic acid (MPA) at concentration ranges normally seen in stable renal transplant patients). At higher mycophenolic acid glucuronide (MPAG) concentrations (e.g., in patients with renal impairment or delayed graft function), binding of MPA may be decreased as a result of competition between MPA and MPAG for binding sites. The mean (+/-SD) apparent volume of distribution of MPA in 12 healthy volunteers is approximately 3.6 (+/-1.5) and 4.0 (+/-1.2) L/kg following intravenous and oral administration, respectively. MPA, at clinically relevant concentrations, is 97% bound to plasma albumin. MPAG is 82% bound to plasma albumin at MPAG concentration ranges that are normally seen in stable renal transplant patients; however, at higher MPAG concentrations (observed in patients with renal impairment or delayed renal graft function), the binding of MPA may be reduced as a result of competition between MPAG and MPA for protein binding. Mean blood to plasma ratio of radioactivity concentrations was approximately 0.6 indicating that MPA and MPAG do not extensively distribute into the cellular fractions of blood. For more Absorption, Distribution and Excretion (Complete) data for MYCOPHENOLATE MOFETIL (9 total), please visit the HSDB record page. Metabolism / Metabolites After both oral and intravenous administration mycophenolate mofetil is entirely metabolized by liver carboxylesterases 1 and 2 to mycophenolic acid (MPA), the active parent drug. It is then metabolized by the enzyme glucuronyl transferase, producing the inactive phenolic glucuronide of MPA (MPAG). The glucuronide metabolite is important, as it is then converted to MPA through enterohepatic recirculation. Mycophenolate mofetil that escapes metabolism in the intestine enters the liver via the portal vein and is transformed to pharmacologically active MPA in the liver cells.N-(2-carboxymethyl)-morpholine, N-(2-hydroxyethyl)-morpholine, and the N-oxide portion of N-(2-hydroxyethyl)-morpholine are additional metabolites of MMF occurring in the intestine as a result of liver carboxylesterase 2 activity. UGT1A9 and UGT2B7 in the liver are the major enzymes contributing to the metabolism of MPA in addition to other UGT enzymes, which also play a role in MPA metabolism. The four major metabolites of MPA are 7-O-MPA-β-glucuronide (MPAG, inactive), MPA acyl-glucuronide (AcMPAG), produced by uridine 5ʹ-diphosphate glucuronosyltransferases (UGT) activities, 7-O-MPA glucoside produced via UGT, and small amounts 6-O-des-methyl-MPA (DM-MPA) via CYP3A4/5 and CYP2C8 enzymes. Following oral and intravenous dosing, mycophenolate mofetil undergoes complete metabolism to MPA /mycophenolic acid/, the active metabolite. Metabolism to MPA occurs presystemically after oral dosing. MPA is metabolized principally by glucuronyl transferase to form the phenolic glucuronide of MPA (MPAG) which is not pharmacologically active. In vivo, MPAG is converted to MPA via enterohepatic recirculation. The following metabolites of the 2- hydroxyethyl-morpholino moiety are also recovered in the urine following oral administration of mycophenolate mofetil to healthy subjects: N-(2-carboxymethyl)-morpholine, N-(2- hydroxyethyl)-morpholine, and the N-oxide of N-(2-hydroxyethyl)-morpholine. Biological Half-Life The average apparent half-life of mycophenolate mofetil is 17.9 (±6.5) hours after oral administration and 16.6 (±5.8) hours after intravenous administration. For mycophenolic acid (MPA):Mean apparent: Approximately 17.9 hours after oral administration and 16.6 hours after intravenous administration. Mean (+/-SD) apparent half-life and plasma clearance of MPA are 17.9 (+/-6.5) hours and 193 (+/-48) mL/min following oral administration and 16.6 (+/-5.8) hours and 177 (+/-31) mL/min following intravenous administration, respectively. Pharmacokinetic Properties [2] Mycophenolate mofetil is well absorbed after oral administration and is rapidly converted to the active metabolite mycophenolic acid. The area under the plasma concentration-time curve (AUC) is generally proportional to dosage; however, there is some interpatient variation in values. The AUC and peak plasma concentration (Cmax) of mycophenolic acid are approximately 50% higher in stable renal transplant patients (>3 months post-transplantation) than in patients during the immediate post-transplant period. Mycophenolic acid is primarily eliminated (≈87%) in the urine as mycophenolic acid glucuronide; 6% is eliminated in the faeces. The mean ‘apparent’ half-life and plasma clearance of mycophenolic acid are 17.9 hours and 11.6 L/h, respectively, after oral administration. The AUC of mycophenolic acid and its glucuronide metabolite were higher in patients with renal impairment than in patients with normal renal function following single dose administration. However, the pharmacokinetic s of mycophenolic mofetil after a single dose are not altered in patients with cirrhosis. There are limited data in children but AUC and Cmax of mycophenolic acid appear to rise with increasing age. |
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
The protein binding of mycophenolic acid, the metabolite of mycophenolate mofetil, is 97% and it is mainly bound to albumin. MPAG, the inactive metabolite, is 82% bound to plasma albumin at normal therapeutic concentrations. At elevated MPAG concentrations due to various reasons, including renal impairment, the binding of MPA may be decreased due to competition for binding. Mycophenolate mofetil (the morpholinoethyl ester of mycophenolic acid) inhibits de novo purine synthesis via the inhibition of inosine monophosphate dehydrogenase. Its selective lymphocyte antiproliferative effects involve both T and B cells, preventing antibody formation. Mycophenolate mofetil has immunosuppressive effects alone, but is used most commonly in combination with other immunosuppressants. Mycophenolate mofetil, in combination with cyclosporin and corticosteroids, has been studied in large, randomised clinical trials involving nearly 1500 renal allograft transplant recipients. These trials demonstrated that mycophenolate mofetil is significantly more effective in reducing treatment failure and acute rejection episodes than placebo or azathioprine. Additionally, mycophenolate mofetil may be able to reduce the occurrence of chronic rejection. Mycophenolate mofetil is relatively well tolerated. The most common adverse effect reported is gastrointestinal intolerance; haematological aberrations have also been noted. The reversible cytostatic action of mycophenolate mofetil allows for dose adjustment or discontinuation, preventing serious toxicity or an overly suppressed immune system. Cytomegalovirus tissue invasive disease and the development of malignancies are concerns that merit evaluation in long term follow-up studies. Mycophenolate mofetil does not cause the adverse effects typically associated with other commercially available immunosuppressant medications such as nephrotoxicity, hepatotoxicity, hypertension, nervous system disturbances, electrolyte abnormalities, skin disorders, hyperglycaemia, hyperuricaemia, hypercholesterolaemia, lipid disorders and structural bone loss. Based on preliminary information, a positive benefit-risk ratio has been demonstrated with the use of mycophenolate mofetil in the prophylaxis of rejection in cadaveric renal allograft transplantation. Data from studies in other types of organ transplants are promising, but are too limited to draw clear conclusions. Long term follow-up studies are required to confirm these observations. Although mycophenolate mofetil is expensive, the beneficial effects on the reduction of rejection, treatment failure and related expenses suggest that it is most likely to be cost effective.[1] Tolerability [2] Rates of adverse events associated with mycophenolate mofetil appear to be dose related: 2 g/day is generally better tolerated than 3 g/day. Gastrointestinal (diarrhoea, vomiting), haematological and lymphatic (leucopenia, anaemia), and infectious (sepsis, opportunistic infections) events are most common. Diarrhoea and sepsis (most commonly cytomegalovirus viraemia) were slightly more common in patients receiving mycophenolate mofetil than in those receiving azathi-oprine. There was also an increased proportion of patients with leucopenia after treatment with mycophenolate mofetil 3 g/day compared with azathioprine treatment. The overall risk of malignancies associated with mycophenolate mofetil was similar to that of azathioprine. |
| 参考文献 |
|
| 其他信息 |
Compound derived from Penicillium stoloniferum and related species. It blocks de novo biosynthesis of purine nucleotides by inhibition of the enzyme inosine monophosphate dehydrogenase (IMP DEHYDROGENASE). Mycophenolic acid exerts selective effects on the immune system in which it prevents the proliferation of T-CELLS, LYMPHOCYTES, and the formation of antibodies from B-CELLS. It may also inhibit recruitment of LEUKOCYTES to sites of INFLAMMATION.
See also: Mycophenolic Acid (has active moiety); Mycophenolate Mofetil (is salt form of). In summary, our results indicate that MMF: (i) inhibits the secretion of TNF-α, IL-1β and NO of microglial cells; (ii) inhibits TNF-α secretion of astrocytes; (iii) suppresses the proliferation of microglial cells and astrocytes; (iv) has no direct neuroprotective effects on cultured, excitotoxically injured hippocampal neurones; and (v) acts by inhibiting glial IMPDH. Against a background of promising animal experiments and open label clinical trials on the use of MMF in various CNS disorders, MMF seems to be a promising candidate for further investigations on the treatment of acute brain and spinal cord pathologies.[2] MMF and DCZ, which target T lymphocytes, inhibit inflammatory activity, and thus prevent the development of skin fibrosis in early stages of a mouse model of BLM‐induced scleroderma. However, MMF is more effective at suppressing fibroblastic activation than in DCZ, and has a more prominent antifibrotic effect. These results support the assumption that T lymphocytes play an important role in the pathogenesis of scleroderma, and that suppression of T lymphocytes may be an effective strategy for treatment of human scleroderma, when started during the early stages of the disease. However, targeting T lymphocytes alone may not be an adequate treatment approach for scleroderma.[3] |
| 分子式 |
C23H32CLNO7
|
|---|---|
| 分子量 |
469.96
|
| 精确质量 |
469.187
|
| 元素分析 |
C, 58.78; H, 6.86; Cl, 7.54; N, 2.98; O, 23.83
|
| CAS号 |
116680-01-4
|
| 相关CAS号 |
Mycophenolate Mofetil;128794-94-5
|
| PubChem CID |
6441022
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.222g/cm3
|
| 沸点 |
637.6ºC at 760mmHg
|
| 闪点 |
339.4ºC
|
| 蒸汽压 |
7.51E-17mmHg at 25°C
|
| 折射率 |
1.557
|
| LogP |
3.263
|
| tPSA |
94.53
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
10
|
| 重原子数目 |
32
|
| 分子复杂度/Complexity |
646
|
| 定义原子立体中心数目 |
0
|
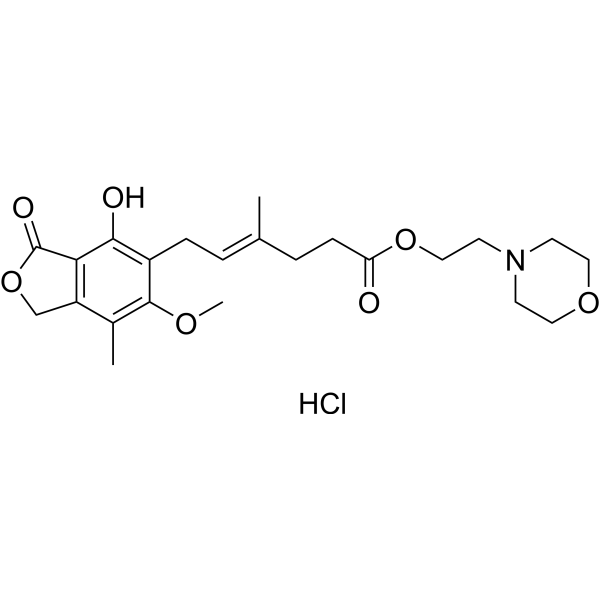
| SMILES |
O=C(OCCN1CCOCC1)CC/C(C)=C/CC2=C(O)C3=C(COC3=O)C(C)=C2OC.[H]Cl
|
| InChi Key |
OWLCGJBUTJXNOF-HDNKIUSMSA-N
|
| InChi Code |
InChI=1S/C23H31NO7.ClH/c1-15(5-7-19(25)30-13-10-24-8-11-29-12-9-24)4-6-17-21(26)20-18(14-31-23(20)27)16(2)22(17)28-3;/h4,26H,5-14H2,1-3H3;1H/b15-4+;
|
| 化学名 |
2-morpholin-4-ylethyl (E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1H-2-benzofuran-5-yl)-4-methylhex-4-enoate;hydrochloride
|
| 别名 |
Mycophenolate mofetil hydrochloride; 116680-01-4; Mycophenolate mofetil HCl; UNII-UXH81S8ZVB; UXH81S8ZVB; RS 61443-190; 2-(4-Morpholinyl)ethyl ester (E)-6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-4-hexenoic acid, hydrochloride; 2-Morpholinoethyl (E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-5-phthalanyl)-4-methyl-4-hexenoate hydrochloride;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1278 mL | 10.6392 mL | 21.2784 mL | |
| 5 mM | 0.4256 mL | 2.1278 mL | 4.2557 mL | |
| 10 mM | 0.2128 mL | 1.0639 mL | 2.1278 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。