| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| 1g |
|
||
| 25g |
|
||
| Other Sizes |
| 体外研究 (In Vitro) |
Triglycidyl isocyanurate(0–30 μM;48 小时)可抑制培养中的人非小细胞肺癌球体的生长,并导致 A549、H460 和 H1299 细胞的肿瘤球逐渐缩小[1]。异氰脲酸三缩水甘油酯(0-30 μM;48 小时)可降低 A549、H460 和 H1299 肿瘤球的 Akt1/2/3 和磷酸化 Aktser473/474/472 的表达;然而,只有 A549 和 H460 肿瘤球表现出明显的 PARP 和 procaspase-3 裂解以及出现的活性 caspase-3 片段[1]。异氰脲酸酯三缩水甘油酯
|
|---|---|
| 体内研究 (In Vivo) |
皮下注射,异氰尿酸替缩水甘油酯(1.8和3.6 mg/kg;每2-3天一次,共7次;30天)抑制异种移植瘤的形成,对裸鼠体重没有影响[2 ]。
|
| 细胞实验 |
细胞活力测定[1]
细胞类型: A549、H460 和 H1299 细胞 测试浓度: 0 μM; 5μM; 10μM; 30 μM 孵育时间:48小时 实验结果:在软琼脂中抑制肿瘤细胞生长。 蛋白质印迹分析[1] 细胞类型: A549、H460 和 H1299 细胞 测试浓度: 0 μM; 5μM; 10μM; 30 μM 孵育时间: 48 小时 实验结果: 抑制 akt1/2/3 表达和 p-aktser473/474/472 表达A549、H460 和 H1299 肿瘤球 |
| 动物实验 |
Animal/Disease Models: Female nu/nu (nude) mice with Huh7 cells subcutaneously (sc) injected into the dorsal area[2]
Doses: 1.8 mg/kg and 3.6 mg/kg Route of Administration: subcutaneous (sc)injection; every 2 –3 days for total seven times; 30 days Experimental Results: Inhibited the growth of xenograft tumors. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
In an oral (gavage) study in mice, at least 17% of the administered dose was absorbed within 24 hr, with blood analysis indicating that the absorption of triglycidyl isocyanurate administered in aqueous solution was twice that of triglycidyl isocyanurate in sesame oil. Triglycidyl isocyanurate was distributed to the liver, stomach, and testes (the only tissues studied). The only available human data are from clinical trials with alpha-triglycidyl isocyanurate (intravenous administration), which indicate that alpha-triglycidyl isocyanurate has ... total body clearance of 5.7 L/min. Less than 1% of the administered dose was recovered unchanged in urine within 24 hr. In oral (gavage) and intravenous studies with [14C]alpha-triglycidyl isocyanurate in rabbits, the radioactivity recovered in urine within 24 hr was approximately 30% and 60-70%, respectively. Metabolism / Metabolites In an oral (gavage) study in mice, ... blood plasma analysis indicated that triglycidyl isocyanurate was metabolized by hydrolysis to the diol diepoxide, the bis-diol epoxide, and the fully hydrolysed tris-diol, with no free triglycidyl isocyanurate detected 8 hr after treatment. In in vitro studies, rapid hydrolysis of triglycidyl isocyanurate involving the enzyme epoxide hydrolase was observed in mouse liver preparations. Hydrolysis was also observed in rat liver preparations but not in rat lung preparations. Microsomal epoxide hydrolase activity with triglycidyl isocyanurate as substrate measured in two human livers obtained from kidney donors was found to be greater than the activity in rat liver. ... Metabolism of TGIC involves hydrolysis of the epoxy functions, leading to the formation of the trisdiol derivative, and is promoted by hepatic but not pulmonary epoxide hydrolase. Non-enzymatic hydrolysis of the epoxy functions occurs under conditions of low pH. Further mechanisms for the metabolism of TGIC have not been investigated. Excretion of TGIC and/or its metabolites is largely via the urine. Urinary metabolites of TGIC have not been identified. Biological Half-Life In ... intravenous studies with [(14)C]alpha-triglycidyl isocyanurate in rabbits, ... the half-life of triglycidyl isocyanurate in the blood was <5 min. The only available human data are from clinical trials with alpha-triglycidyl isocyanurate (intravenous administration), which indicate that alpha-triglycidyl isocyanurate has a mean half-life in the blood of approximately 1 min ... . |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Triglycidyl isocyanurate (TGT) is a white solid. TGT is used as a crosslinking agent in polymer synthesis, additive in plastic, rubber, and adhesives, hardener in polyester powder coatings, in protective coatings of electronic devices, in top-coated and solder-resistant inks. HUMAN STUDIES: The reported health effects in humans are contact dermatitis and respiratory sensitization. It may also cause serious eye damage. TGT did not induce chromosomal aberrations in human lymphocytes at concentrations up to 2500 ng/mL. Only one aberration was reported at each of the two higher concentrations of 5000 and 10,000 ng/mL. Human trials were performed during clinical development of alpha-TGT as an antitumor agent. In these studies, alpha-TGT was administered iv to cancer patients at doses up to 900 mg/kg bw using a variety of dosing regimes. Toxic signs included myelosuppression, nausea and vomiting, and, rarely, alopecia and leucopenia at high doses (>600 mg/kg bw). Owing to its severe local toxicity (thrombophlebitis) at the site of injection, the use of alpha-TGT as an antitumor agent was not pursued. ANIMAL STUDIES: Acute animal toxicity studies reveal that TGT is toxic by oral and inhalation routes of exposure but has low acute dermal toxicity. TGT produces serious eye irritation. It is a skin sensitizer but not a skin irritant. Short-term repeated dose studies revealed renal, lung, gastric/duodenal, and sperm cell damage. In a subchronic toxicity/fertility study conducted in rats, a dose-dependent reduction in the number of spermatozoa was the only effect observed at concentrations of up to 100 ppm TGT in the diet. The chemical has produced positive results in a range of in vitro genotoxicity studies (gene mutation in bacterial and mammalian cells, unscheduled DNA synthesis, sister chromatid exchanges, and chromosomal aberration assays). Genotoxic effects have also been observed in vivo in somatic (bone marrow) cells and germ cells in the testes. Genotoxicity studies have revealed that the inhalation of TGT produces cytotoxicity and chromosomal aberrations in mouse spermatogonia. A 13-week dietary study in rats has indicated no effects on male fertility. Toxicity Data LC50 (rat) > 650 mg/m3/4h Non-Human Toxicity Values LD50 Rat oral 188-715 mg/kg bw LD50 Rat dermal >2000 mg/kg bw |
| 参考文献 | |
| 其他信息 |
Tris(2,3-epoxypropyl)isocyanurate is a white crystalline solid. (NTP, 1992)
Teroxirone is a triazene triepoxide with antineoplastic activity. Teroxine alkylates and cross-links DNA, thereby inhibiting DNA replication. (NCI04) |
| 分子式 |
C12H15N3O6
|
|---|---|
| 分子量 |
297.26
|
| 精确质量 |
297.096
|
| CAS号 |
2451-62-9
|
| 相关CAS号 |
28825-96-9
|
| PubChem CID |
17142
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.6±0.1 g/cm3
|
| 沸点 |
501.1±15.0 °C at 760 mmHg
|
| 熔点 |
95-98°C
|
| 闪点 |
256.9±20.4 °C
|
| 蒸汽压 |
0.0±1.3 mmHg at 25°C
|
| 折射率 |
1.635
|
| LogP |
-2.77
|
| tPSA |
103.59
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
21
|
| 分子复杂度/Complexity |
416
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
OUPZKGBUJRBPGC-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C12H15N3O6/c16-10-13(1-7-4-19-7)11(17)15(3-9-6-21-9)12(18)14(10)2-8-5-20-8/h7-9H,1-6H2
|
| 化学名 |
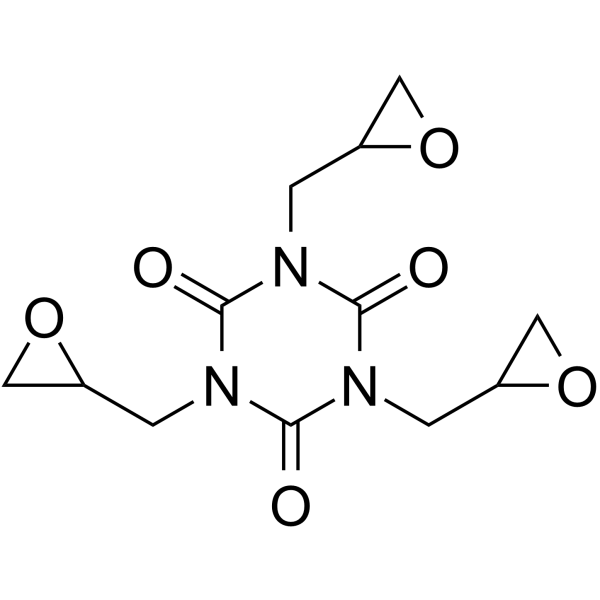
1,3,5-tris(oxiran-2-ylmethyl)-1,3,5-triazinane-2,4,6-trione
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : 50 mg/mL (168.20 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.3641 mL | 16.8203 mL | 33.6406 mL | |
| 5 mM | 0.6728 mL | 3.3641 mL | 6.7281 mL | |
| 10 mM | 0.3364 mL | 1.6820 mL | 3.3641 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。