| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 体外研究 (In Vitro) |
Cangrelor对 hP2Y12 受体的 pKb 为 8.6–9.2[3]。
Cangrelor是唯一有效的静脉直接电位二磷酸腺苷 (ADP) P2Y12 受体钳抗剂[1]。坎格勒四钠的 hP2Y12 受体 pKb 为 8.6-9.2[3]。
Cangrelor是一种选择性、快速可逆的P2Y12血小板受体抑制剂,可直接阻断二磷酸腺苷(ADP)诱导的血小板活化和聚集,并在五分钟内达到90%的血小板抑制水平[1]。 |
|---|---|
| 体内研究 (In Vivo) |
除了显着降低 BLM 诱导的炎症细胞因子产生(PF4、CD40 L 和 MPO)外,Cangrelor (10 mg/kg) 还可以减少外周血小板、中性粒细胞和血小板中性粒细胞,以及肺纤维化和血小板中性粒细胞BLM 治疗小鼠血液中的积累[2]。
研究人员报告称,坎格雷是一种非特异性GPR17拮抗剂,通过抑制小鼠巨噬细胞炎症,部分缓解了肺纤维化。Cangrelor也是一种众所周知的抗血小板药物。为了测试坎格雷是否部分通过抑制血小板来减轻肺纤维化,使用博莱霉素(BLM)诱导C57BL/6 J小鼠的肺纤维化。我们发现,坎格雷(10mg/kg)不仅显著降低了BLM诱导的炎性细胞因子(PF4、CD40L和MPO)的释放,还降低了BLP治疗小鼠纤维化肺和外周血中血小板、中性粒细胞和血小板-中性粒细胞聚集体的增加。此外,坎格雷降低了BLM治疗的小鼠肺部CD40和MPO双阳性中性粒细胞的数量以及CD40的表达水平。基于这些结果,得出结论,坎格雷减轻了BLM诱导的小鼠肺部炎症和肺纤维化,部分是通过抑制血小板活化,从而减少了CD40-CD40L相互作用介导的血小板和中性粒细胞粘附引起的中性粒细胞浸润。Cangrelor可能是治疗肺纤维化的潜在药物。[2] 方法在野生型和P2ry12-/-基因缺陷型小鼠中诱导完全弗氏佐剂(CFA)诱导的慢性炎症性疼痛,并使用强效、直接作用和可逆的P2Y12受体拮抗剂PSB-0739和坎格雷。结果在急性炎症期,CFA诱导的P2ry12-/-小鼠机械性痛觉过敏显著降低,持续14天,后爪中性粒细胞髓过氧化物酶活性和肿瘤坏死因子(TNF)-α和CXCL1(KC)水平的升高也有所减轻。在第14天,P2ry12-/-小鼠白细胞介素(IL)-1β、IL-6、TNF-α和KC水平的升高减弱。PSB-0739和坎格雷逆转了野生型小鼠的痛觉过敏,但对P2ry12-/-小鼠没有影响,局部应用时PSB-0738也有效。选择性NaV1.8通道拮抗剂A-803467可预防局部和全身PSB-0739的作用,表明NaV1.8渠道参与了抗痛觉过敏作用。在第14天,抗小鼠CD41抗体的血小板耗竭降低了野生型小鼠的痛觉过敏并减弱了促炎细胞因子反应,但在P2ry12-/-小鼠中没有。结论总之,P2Y12受体调节CFA诱导的痛觉过敏和局部炎症反应,血小板P2Y12接收器在慢性炎症阶段参与了这些作用[3]。 |
| 动物实验 |
Animals and reagents [2]
C57BL/6 J mice (male, 6–8 weeks, 22−25 g) were used. The mice are free access to water and food in air-conditioned rooms (23 °C, relative humidity 50 %) on a 12 h light / dark cycle. Four treatments were performed in these mice: sham-operated control (Con, n = 6), cangrelor (Cang, 10 mg/kg, n = 6), bleomycin + saline (BLM, 3 mg/kg, n = 6) and bleomycin + cangrelor (BLM + Cang 10 mg/kg, n = 6). Cangrelor and bleomycin (Hisun Pharmaceutical Co., Ltd., China) were stored at 4 °C and diluted in saline before use. Experimental procedure and cangrelor administration [2] According to the previous report (Zhan et al., 2018, 2018; Tanaka et al., 2017), pulmonary fibrosis was induced by intratracheal administration of bleomycin (BLM, 3 mg/kg) in C57BL/6 J mice, and cangrelor (10 mg/kg) was administrated via subcutaneous injection. BLM was administrated on day 0, the treatment of cangrelor was started 2 days before BLM administration and lasted for 16 days (once per day). On day 14, the mice were sacrificed by cervical dislocation after the pulmonary resistance was determined. The bronchoalveolar lavage fluid (BALF) was collected from right lung, then the right lung tissues were stored at -80 °C for Western blotting analysis and quantitative reverse-transcription polymerase chain reaction (qRT-PCR). The left lung tissues were fixed in 10 % formaldehyde for histological inspection. The blood and BALF were collected for flow cytometry and ELISA assay. Mice were treated with P2Y12R antagonists, or with their vehicle (sterile saline), intraperitoneally ([dichloro‐[[[(2R,3S,4R,5R)‐3,4‐dihydroxy‐5‐[6‐(2‐methylsulfanylethylamino)‐2‐(3,3,3‐trifluoropropylsulfanyl)purin‐9‐yl]oxolan‐2‐yl]methoxy‐hydroxyphosphoryl]‐oxyhydroxyphosphoryl]methyl]phosphonic‐acid, cangrelor , 3 mg kg−1; The Medicines Company, Parsippany, NJ, USA), intraplantarly or intrathecally (1‐amino‐4‐[4‐phenylamino‐3‐sulfophenylamino]‐9,10‐dioxo‐9,10‐dihydroanthracene‐2‐sulfonate, PSB‐0739, 0.3 mg kg−1, selective P2Y12R antagonist synthesized by Y. Baqi and C. E. Müller). on days 3, 4, 7, 10 and 14 after CFA injection. The doses were chosen on the basis of our previous experiments: the pKB values of PSB‐0739 and cangrelor at human P2Y12Rs (hP2Y12Rs) were 9.8 and 8.6, respectively, whereas, in the doses applied in the present study (PSB‐0739, 0.3 mg kg−1 intrathecally; cangrelor , 3 mg kg−1 intraperitoneally), they reversed acute inflammatory pain for up to 96 h. Taking into account that the approximate blood volume of a 25‐mg mice is 1700 μL, these doses correspond to 5 μm and 50 μm, indicating maximal target inhibition. As a reference compound, aspirin, an alternative platelet antagonist, was used at a low dose (2‐acetyloxybenzoic acid, 20 mg kg−1 intraperitoneally). The mechanonociceptive thresholds of hind paws were measured 15 min or 30 min after intrathecal/intraperitoneal or intraplantar injections, with the exception of day 3, when PWT measurements were performed before drug administration. 5‐(4‐chlorophenyl)‐N‐(3,5‐dimethoxyphenyl)‐2‐furancarboxamide (A‐803467, 30 mg kg−1), a potent and selective NaV1.8 sodium channel antagonist or its vehicle (polyethylene glycol and dimethyl sulfoxide [9 : 1]) was administered intraperitoneally 5 min before the respective PSB‐0739/saline injection. The dose of A‐803467 was chosen on the basis of a previous study, and a submaximal dose (30 mg kg−1 intraperitoneally) in the reduction of mechanical allodynia was selected to reveal any additive interactions between PSB‐0739 and A‐803467. In some experiments, paw edema was also volumetrically quantified by plethysmometry (7140; Ugo Basile). [3] Assessment of platelet CD62P levels by flow cytometry [3] To investigate how P2Y12R antagonists and antiplatelet agents administered via different routes altered platelet activation, we measured ADP‐induced changes in platelet CD62P levels ex vivo, in platelet‐rich plasma (PRP) samples. Wild‐type mice were treated with PSB‐0739 (0.3 mg kg−1 intrathecally), cangrelor (3 mg kg−1 intraperitoneally), aspirin (20 mg kg−1 intraperitoneally), or their vehicle. Blood samples were taken directly from the vena cava of anesthetized mice 15 min or 30 min after the treatment. Apyrase (1 U mL−1) was added to the samples to prevent ADP receptor desensitization. After 10 min of centrifugation at 150 × g, PRP was collected. Platelet activation was induced by ADP (500 μm), and changes in platelet CD62P levels were assessed after 60 min of incubation. Platelets were stained with anti‐human/mouse CD62P antibody for 10 min. Samples were acquired with a BD FACSVerse machine, and analyzed with BD facsuite software. Changes in CD62P mean fluorescence intensity values were determined on CD42d‐positive platelets. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following IV administration of [3H] cangrelor, 58% of radioactivity was recovered in urine. The remaining 35% of radioactivity was in feces, presumably following biliary excretion. In a study in healthy volunteers administration at a dose of 30 mcg/kg bolus plus 4 mcg/kg/min showed a volume of distribution of 3.9 L. The mean clearance is about 43.2 L/h. /MILK/ It is not known whether Kengreal is excreted in human milk. Following IV administration of 3(H) Kengreal 58% of radioactivity was recovered in urine. The remaining 35% of radioactivity was in feces, presumably following biliary excretion. The average elimination half-life of Kengreal is about 3-6 minutes. In a study in healthy volunteers, Kengreal administration at a dose of 30 ug/kg bolus plus 4 mcg/kg/min showed a volume of distribution of 3.9 L. Plasma protein binding of Kengreal is about 97-98%. Metabolism / Metabolites Cangrelor is deactivated rapidly in the circulation by dephosphorylation to its primary metabolite, a nucleoside, which has negligible anti-platelet activity. Cangrelor's metabolism is independent of hepatic function and it does not interfere with other drugs metabolized by hepatic enzymes. Kengreal is deactivated rapidly in the circulation by dephosphorylation to its primary metabolite, a nucleoside, which has negligible anti-platelet activity. Kengreal's metabolism is independent of hepatic function and it does not interfere with other drugs metabolized by hepatic enzymes. Biological Half-Life The average elimination half-life of cangrelor is about 3-6 minutes. Following IV administration of 3(H) Kengreal, ... elimination half-life of Kengreal is about 3-6 minutes. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Cangrelor is a platelet aggregation inhibitor and purinergic P2Y receptor antagonist. HUMAN STUDIES: Cangrelor is a potent intravenous platelet P2Y12 receptor antagonist with rapid onset and offset of action. In patients undergoing percutaneous coronary interventions (PCI), compared with control, cangrelor (30 ug/kg bolus, followed immediately by a 4 ug/kg per minute infusion for 2-4 hr or until the conclusion of the index PCI, whichever was longer) reduces periprocedural thrombotic complications without an increase in major bleeding complications, although minor bleeding is increased. In a large clinical trial program of patients undergoing PCI, cangrelor overdosing was rare and not associated with an increase in bleeding complications, an observation that may be attributed to its very short-half life and rapid offset of action. Platelet P2Y12 receptor expression is significantly increased and the receptor is constitutively activated in patients with type 2 diabetes mellitus, which contributes to platelet hyperactivity and limits antiplatelet drug efficacy in type 2 diabetes mellitus. Cangrelor was non-mutagenic and non-clastogenic in genetic toxicology studies, including chromosome aberration assay in human peripheral lymphocytes. ANIMAL STUDIES: Cangrelor had no significant effect on male or female rats fertility treated for 28 days, or on early embryonic development. In embryo-fetal development studies in rats, cangrelor produced dose-related fetal growth retardation characterized by increased incidences of incomplete ossification and unossified hind limb metatarsals. In rabbits, cangrelor was associated with increased incidences of abortion and intrauterine losses, as well as fetal growth retardation. Cangrelor was non-mutagenic and non-clastogenic in genetic toxicology studies, including in vitro bacterial gene mutation assay, mouse lymphoma thymidine kinase assay, and in vivo bone marrow micronucleus assay in mice. Hepatotoxicity In several large clinical trials, serum ALT elevations were no more frequent with cangrelor therapy than with placebo [9% vs 12%] or with comparator arms [6.6% vs 6.8%] and no cases of clinically apparent liver injury with jaundice were reported. In addition, since marketing and release, there have been no published reports of clinically apparent liver injury or jaundice associated with cangrelor therapy and hepatotoxicity is not mentioned in the product label. Likelihood score: E (unlikely cause of clinically apparent liver injury). Protein Binding about 97-98%. Interactions BACKGROUND: Agents that act as antagonists at P2Y(12) ADP receptors on platelets are in use (clopidogrel), and in development for use (cangrelor and prasugrel), in patients with cardiovascular disease. Cangrelor is a direct-acting reversible antagonist being developed for short-term infusion; clopidogrel and prasugrel are oral prodrugs that provide irreversible inhibition via transient formation of active metabolites. At the cessation of cangrelor infusion, patients are likely to receive clopidogrel or prasugrel as a means of maintaining antiplatelet therapy. OBJECTIVES: To apply an experimental in vitro approach to investigate the possibility that cangrelor influences the ability of the active metabolites of clopidogrel and prasugrel to inhibit ADP-mediated platelet function. METHODS: The effects of cangrelor and the active metabolites of clopidogrel (C-AM) and prasugrel (P-AM) on platelet function were assessed by ADP-induced platelet P-selectin expression in whole blood. The method involved rapid removal of the antagonists by dilution, and measurement of residual platelet inhibition. RESULTS: Cangrelor, C-AM and P-AM markedly inhibited P-selectin expression. The effect of cangrelor, but not of C-AM and P-AM, was reversible following antagonist removal. Preincubation of blood with cangrelor prior to addition of C-AM or P-AM reduced the ability of metabolites to irreversibly antagonize P2Y(12). Irreversible inhibition was maintained when blood was preincubated with metabolites prior to cangrelor. CONCLUSIONS: Cangrelor influences the ability of the active metabolites of clopidogrel or prasugrel to inhibit platelet function irreversibly. Careful consideration should be given to the timing of administration of an oral P2Y(12) antagonist following cangrelor infusion. Concomitant administration of cangrelor with the thienopyridine antiplatelet drugs clopidogrel or prasugrel decreases the antiplatelet effect of clopidogrel and prasugrel by blocking P2Y12-receptor binding of the active metabolites of these drugs. Oral maintenance antiplatelet therapy with clopidogrel or prasugrel should not be administered until the cangrelor infusion has been discontinued. |
| 参考文献 |
|
| 其他信息 |
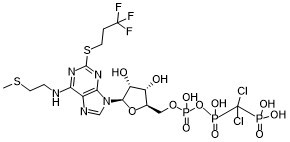
Cangrelor is a nucleoside triphosphate analogue that is 5'-O-[({[dichloro(phosphono)methyl](hydroxy)phosphoryl}oxy)(hydroxy)phosphoryl]adenosine carrying additional 2-(methylsulfanyl)ethyl and (3,3,3-trifluoropropyl)sulfanyl substituents at positions N6 and C2 respectively. Used (in the form of its tetrasodium salt) as an intravenous antiplatelet drug that prevents formation of harmful blood clots in the coronary arteries. It has a role as a platelet aggregation inhibitor and a P2Y12 receptor antagonist. It is a nucleoside triphosphate analogue, an organofluorine compound, an aryl sulfide, an organochlorine compound, a secondary amino compound and an adenosine 5'-phosphate. It is a conjugate acid of a cangrelor(4-).
Cangrelor is an intravenous, direct-acting, reversible P2Y12 inhibitor for patients undergoing percutaneous coronary intervention (PCI) who have not been yet treated by oral P2Y12 inhibitors. An advantage Cangrelor provides over oral P2Y12 inhibitors (such as prasugrel, ticagrelor, and clopidogrel) is that it is an active drug not requiring metabolic conversion therefore providing a rapid onset and offset of action. Cangrelor was approved by the FDA in June 2015 for intravenous application. Cangrelor is a P2Y12 Platelet Inhibitor. The mechanism of action of cangrelor is as a P2Y12 Receptor Antagonist. The physiologic effect of cangrelor is by means of Decreased Platelet Aggregation. Cangrelor is an intravenously administered antiplatelet drug that is used at the time of cardiac surgery or percutaneous coronary intervention to decrease the risk of myocardial infarction and maintain artery and stent patency. Cangrelor has not been linked to serum enzyme abnormalities or to clinically apparent liver injury, but its clinical use has been limited. Cangrelor is an inhibitor of the platelet adenosine diphosphate (ADP) P2Y12 receptor (P2Y12R), with antiplatelet activity. Upon administration, cangrelor selectively and reversibly binds to P2Y12R, and blocks the platelet signaling pathway. This inhibits the activation of the glycoprotein complex GPIIb/IIIa, fibrinogen binding to platelets and platelet adhesion and aggregation. Drug Indication For use as an adjunct to percutaneous coronary intervention (PCI) for reducing the risk of periprocedural myocardial infarction (MI), repeat coronary revascularization, and stent thrombosis (ST) in patients in who have not been treated with a P2Y12 platelet inhibitor and are not being given a glycoprotein IIb/IIIa inhibitor. FDA Label Kengrexal, co-administered with acetylsalicylic acid (ASA), is indicated for the reduction of thrombotic cardiovascular events in adult patients with coronary artery disease undergoing percutaneous coronary intervention (PCI) who have not received an oral P2Y12 inhibitor prior to the PCI procedure and in whom oral therapy with P2Y12 inhibitors is not feasible or desirable. Mechanism of Action Cangrelor is a selective, reversible, P2Y12 platelet receptor antagonist which inhibits ADP platelet aggregation. ADP is typically released by damaged blood vessels, red blood cells, and/or platelets due to agonists stimulating platelet activity. ADP binds to P2Y12 to stimulate and complete platelet aggregation by inhibiting adenylyl cyclase by a Gi protein, thus potentiating dense granule secretion and increasing coagulation activity. Cangrelor acts on the same target as oral irreversible inhibitors clopidogrel and ticlopidine and has a similar mechanism of action, but is reversible and provides a fast onset and offset of action. Cangrelor is a direct P2Y12 platelet receptor inhibitor that blocks ADP-induced platelet activation and aggregation. Cangrelor binds selectively and reversibly to the P2Y12 receptor to prevent further signaling and platelet activation. Therapeutic Uses Platelet Aggregation Inhibitors; Purinergic P2Y Receptor Antagonists /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Cangrelor is included in the database. Kengreal is indicated as an adjunct to percutaneous coronary intervention (PCI) to reduce the risk of periprocedural myocardial infarction (MI), repeat coronary revascularization, and stent thrombosis (ST) in patients who have not been treated with a P2Y12 platelet inhibitor and are not being given a glycoprotein IIb/IIIa inhibitor. /Included in US product label/ Drug Warnings The most common adverse effect of cangrelor reported in clinical trials was bleeding. Cases of transient dyspnea were also reported during clinical trials. Hypersensitivity reactions (e.g., anaphylaxis, bronchospasm, angioedema, stridor) have been reported with cangrelor therapy. Like other antiplatelet agents, cangrelor increases the risk of bleeding, which may be serious. In the CHAMPION PHOENIX trial, bleeding events of all severities were somewhat more common with cangrelor than with clopidogrel. In clinical trials, bleeding events in patients receiving cangrelor were mild, generally consisting of hematoma, ecchymosis, and oozing at the puncture site. In the CHAMPION PHOENIX trial, the rate of severe bleeding (per the Global Use of Strategies to Open Occluded Coronary Arteries [GUSTO] criteria) was not substantially increased by cangrelor, although the rate of major bleeding according to more sensitive criteria (Acute Catheterization and Urgent Intervention Triage Strategy [ACUITY]) was substantially higher with cangrelor than with clopidogrel (4.3 versus 2.5%). The increase in major bleeding with the ACUITY criteria was attributable to a greater incidence of hematoma at the site of vascular access in patients receiving cangrelor. Cangrelor should not be used in patients with substantial active bleeding. The antiplatelet effects of cangrelor are negligible 1 hour after discontinuance of the infusion. For more Drug Warnings (Complete) data for Cangrelor (11 total), please visit the HSDB record page. Cangrelor tetrasodium is an organic sodium salt that is the tetrasodium salt of cangrelor. Used as an intravenous antiplatelet drug that prevents formation of harmful blood clots in the coronary arteries. It has a role as a platelet aggregation inhibitor and a P2Y12 receptor antagonist. It contains a cangrelor(4-). Cangrelor Tetrasodium is the tetrasodium salt form of cangrelor, an inhibitor of the platelet adenosine diphosphate (ADP) P2Y12 receptor (P2Y12R), with antiplatelet activity. Upon administration, cangrelor selectively and reversibly binds to P2Y12R, and blocks the platelet signaling pathway. This inhibits the activation of the glycoprotein complex GPIIb/IIIa, fibrinogen binding to platelets and platelet adhesion and aggregation. Drug Indication Kengrexal, co-administered with acetylsalicylic acid (ASA), is indicated for the reduction of thrombotic cardiovascular events in adult patients with coronary artery disease undergoing percutaneous coronary intervention (PCI) who have not received an oral P2Y12 inhibitor prior to the PCI procedure and in whom oral therapy with P2Y12 inhibitors is not feasible or desirable. Due to the increasing number of patients on antiplatelet therapy for cardiovascular and neurological conditions, it can be challenging to manage these patients peri-operatively. It is critical to prevent ischemia and thrombosis, and at the same time decrease the risk of bleeding, necessitating the need for a successful bridging therapy. Cangrelor looks promising as a bridging therapy with its distinctive pharmacokinetic profile with fast activity and easy reversibility. However, large prospective studies are required to delineate clear guidelines to identify the patient population that would receive maximum benefit from bridging antiplatelet therapy, determine optimal dosing and titration, monitoring therapy, and manage adverse events. Although guidelines recommend IV bridge therapy in these settings, no agent currently has FDA-approval for this indication and positive, randomized controlled data for GPIs in this setting is also lacking. Cangrelor bridging therapy appears to have advantages over the previous standard using GPIs due to its faster offset and non-renal clearance. Future research is warranted for use of cangrelor in special populations, such as those with CAD on DAPT as a bridge to LVAD implantation. Overall, in this complex era of advancing medical technologies, therapies such as cangrelor may mitigate thrombotic and bleeding risks in the peri-operative period. [1] |
| 分子式 |
C24H27N3O4S
|
|---|---|
| 分子量 |
776.359300000001
|
| 精确质量 |
774.948
|
| 元素分析 |
C, 26.30; H, 3.25; Cl, 9.13; F, 7.34; N, 9.02; O, 24.73; P, 11.97; S, 8.26
|
| CAS号 |
163706-06-7
|
| 相关CAS号 |
Cangrelor tetrasodium;163706-36-3
|
| PubChem CID |
9854012
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
2.087g/cm3
|
| 沸点 |
979.004ºC at 760 mmHg
|
| 闪点 |
545.882ºC
|
| 蒸汽压 |
0mmHg at 25°C
|
| 折射率 |
1.722
|
| LogP |
2.923
|
| tPSA |
335.94
|
| 氢键供体(HBD)数目 |
7
|
| 氢键受体(HBA)数目 |
21
|
| 可旋转键数目(RBC) |
15
|
| 重原子数目 |
44
|
| 分子复杂度/Complexity |
1140
|
| 定义原子立体中心数目 |
4
|
| SMILES |
CSCCNC1=C2C(=NC(=N1)SCCC(F)(F)F)N(C=N2)[C@H]3[C@@H]([C@@H]([C@@H](COP(=O)(O)OP(=O)(C(Cl)(Cl)P(=O)(O)O)O)O3)O)O
|
| InChi Key |
PAEBIVWUMLRPSK-IDTAVKCVSA-N
|
| InChi Code |
InChI=1S/C17H25Cl2F3N5O12P3S2/c1-43-5-3-23-12-9-13(26-15(25-12)44-4-2-16(20,21)22)27(7-24-9)14-11(29)10(28)8(38-14)6-37-42(35,36)39-41(33,34)17(18,19)40(30,31)32/h7-8,10-11,14,28-29H,2-6H2,1H3,(H,33,34)(H,35,36)(H,23,25,26)(H2,30,31,32)/t8-,10-,11-,14-/m1/s1
|
| 化学名 |
(dichloro((((((2R,3S,4R,5R)-3,4-dihydroxy-5-(6-((2-(methylthio)ethyl)amino)-2-((3,3,3-trifluoropropyl)thio)-9H-purin-9-yl)tetrahydrofuran-2-yl)methoxy)(hydroxy)phosphoryl)oxy)(hydroxy)phosphoryl)methyl)phosphonic
acid
|
| 别名 |
AR-C69931; AR C69931; MXAR C69931; 163706-06-7; Kengreal; AR-C69931XX; Cangrelor free acid; UNII-6AQ1Y404U7; 6AQ1Y404U7; ARL69931; AR-C69931MX; ARC69931MX; ARC69931
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.2881 mL | 6.4403 mL | 12.8806 mL | |
| 5 mM | 0.2576 mL | 1.2881 mL | 2.5761 mL | |
| 10 mM | 0.1288 mL | 0.6440 mL | 1.2881 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。