| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| Other Sizes |
|
| 靶点 |
VEGFR2 (IC50 = 0.2 nM); VEGFR3 (IC50 = 0.7 nM); c-Kit (IC50 = 14.8 nM); c-Kit (IC50 = 14.8 nM); c-Kit (IC50 = 14.8 nM)
Anlotinib Dihydrochloride primarily targets vascular endothelial growth factor receptor-2 (VEGFR2) with an IC50 < 1 nmol/L; it also inhibits VEGF-induced signaling and proliferation in HUVEC with picomolar IC50 values [1] Anlotinib Dihydrochloride is a multi-target tyrosine kinase inhibitor that potently inhibits VEGFR2/3, FGFR1-4, PDGFR α/β, c-Kit, and Ret [2] |
|---|---|
| 体外研究 (In Vitro) |
体外活性:安罗替尼(原名 AL3818)是一种新型强效多激酶抑制剂,可抑制 VEGFR2/3、FGFR1-4、PDGFRα/β、c-Kit 和 Ret。安罗替尼作为受体酪氨酸激酶 (RTK) 抑制剂,具有潜在的抗肿瘤和抗血管生成活性。安罗替尼在体外显着减少 AN3CA 细胞数量,其特点是突变 FGFR2 蛋白的高表达。每日口服安罗替尼(5 mg/kg)使 55% 的接受治疗的动物出现完全缓解,并且在 29 次治疗后,AN3CA 肿瘤的肿瘤体积和肿瘤重量分别减少了 94% 和 96%。天治疗周期。尽管卡铂和紫杉醇未能改变肿瘤生长,但与单独使用安罗替尼治疗相比,与安罗替尼联合治疗似乎并未表现出更好的效果。 激酶检测:安罗替尼(以前称为 AL3818)是一种新型、有效的多激酶抑制剂,可抑制 VEGFR2 /3、FGFR1-4、PDGFRα/β、c-Kit 和 Ret.细胞检测:AL3818 在体外显着减少 AN3CA 细胞数量,其特征是突变的 FGFR2 蛋白高表达。每日口服 AL3818 (5 mg/kg) 可使 55% 的接受治疗动物出现完全缓解,并且在 29 次治疗后,AN3CA 肿瘤的肿瘤体积减小,肿瘤重量分别减少 94% 和 96%。天治疗周期。尽管卡铂和紫杉醇未能改变肿瘤生长,但与单独使用 AL3818 治疗相比,与 AL3818 的组合似乎并未表现出更好的效果。
盐酸安罗替尼可结合VEGFR2酪氨酸激酶的ATP结合口袋,对VEGFR2具有高选择性和抑制活性(IC50<1 nmol/L),远高于对其他酪氨酸激酶的抑制作用。它以皮摩尔级IC50抑制VEGF诱导的HUVEC信号通路激活和细胞增殖,而直接抑制肿瘤细胞增殖则需要微摩尔浓度。此外,该药物能显著抑制HUVEC的迁移和管形成,抑制大鼠主动脉外植体的微血管出芽,在体外阻断血管生成相关过程 [1] 盐酸安罗替尼在体外具有良好的膜通透性。它对大多数人细胞色素P450酶(CYPs)、尿苷二磷酸葡萄糖醛酸转移酶(UGTs)和转运体的抑制作用较弱,但对CYP3A4和CYP2C9的体外抑制IC50<1μmol/L。药物与血浆蛋白结合率较高,大鼠为97%、犬为96%、人为93% [3] |
| 体内研究 (In Vivo) |
每日口服安罗替尼(5 mg/kg)使 55% 的接受治疗的动物出现完全缓解,并且在 29 次治疗后,AN3CA 肿瘤的肿瘤体积和肿瘤重量分别减少了 94% 和 96%。天治疗周期。尽管卡铂和紫杉醇未能改变肿瘤生长,但与单独使用安罗替尼治疗相比,与安罗替尼的组合似乎并未表现出更好的效果。
安洛替尼在人异种移植物模型中的抗肿瘤疗效[1] 鉴于安洛替尼活性在体外具有令人鼓舞的抗血管生成作用,我们接下来评估了安洛替尼在人类结肠癌癌症SW620异种移植物模型中的体内抗肿瘤潜力。每日一次口服剂量的安洛替尼可引起肿瘤生长的剂量依赖性抑制(图5A,C),与对照组相比,3 mg/kg的剂量可抑制83%的肿瘤生长。相比之下,在该模型中,达到相当疗效所需的舒尼替尼剂量为50 mg/kg。此外,在所有组的实验过程中,安洛替尼对小鼠体重的影响很小(图5B)。我们通过使用内皮细胞标志物CD31的免疫组织化学分析测量提取的肿瘤中的微血管密度,进一步评估了肿瘤血管生成。安洛替尼诱导CD31阳性微血管显著减少,在0.75、1.5和3 mg/kg的剂量下,抑制率分别为48.9%、76.3%和91.2%(图5D)。相比之下,50mg/kg剂量的舒尼替尼抑制了63.6%的微血管密度。 在多种异种移植物模型中进一步研究了安洛替尼的体内抗肿瘤潜力,该模型通过接种具有不同VEGF/VEGFR2 mRNA表达水平的人癌症细胞系(图S1;数据S1)或其他RTK.35,40创建。在所有测试的肿瘤模型中,每日口服一次安洛替尼对肿瘤生长产生了剂量依赖性抑制作用(表2;图6)。在3 mg/kg的剂量下,安洛替尼在U-87MG、Caki-1、Calu-3和SK-OV-3异种移植物中分别抑制了55%、80%、91%和97%的肿瘤生长,在最后一个治疗日进行测量。此外,它在Calu-3和SK-OV-3肿瘤异种移植物模型中都导致了肿瘤消退。在后一种异种移植物模型中,用更高剂量的安洛替尼(6mg/kg)治疗可抑制95%的肿瘤生长;重要的是,在停用安洛替尼后12天内,肿瘤没有反弹。与3 mg/kg的情况一样,6 mg/kg的安洛替尼在Calu-3和SK-OV-3肿瘤异种移植物模型中均导致肿瘤消退。 裸鼠异种移植模型(SW620、U-87 MG、Caki-1、SK-OV-3、Calu-3)中,每日口服盐酸安罗替尼的体内抗肿瘤疗效比舒尼替尼更广、更强,部分模型中可诱导肿瘤消退;同时该药物通过抑制血管生成降低肿瘤组织的血管密度 [1] 晚期难治性实体瘤患者接受盐酸安罗替尼12mg每日一次、2周给药/1周停药(2/1)方案治疗后,20例可评估患者中,3例达到部分缓解,14例病情稳定(其中12例肿瘤负荷缩小),3例疾病进展 [2] 盐酸安罗替尼在大鼠中的口服生物利用度为28%-58%,犬中为41%-77%;大鼠的终末半衰期为5.1±1.6h,犬为22.8±11.0h。药物在大鼠和荷瘤小鼠各组织中的浓度显著高于血浆浓度,细胞色素P450介导的代谢是其主要消除途径 [3] |
| 酶活实验 |
使用 ELISA 可以确定安罗替尼对酪氨酸激酶的抑制活性。 ATP 和酪氨酸激酶在反应缓冲液(50 mmol/L HEPES pH 7.4、50 mmol/L MgCl2、0.5 mmol/L MnCl2、0.2 mmol/L Na 3VO4, 1 mmol/L DTT),在涂有 20 μg/mL Poly(Glu,Tyr)4:1 的 96 孔板中孵育 1 小时37°C。 PY99 抗体孵育后,将 HRP 缀合的抗小鼠 IgG 添加到板中。分析仪:与邻苯二胺溶液反应并添加2N H2SO4终止后,使用Synergy H4 Hybrid读数器测量490 nm处的吸光度。
体外代谢研究[3] 为了鉴定可以介导安洛替尼氧化的人类P450亚型,将各种用NADPH强化的cDNA表达的人类P450酶(CYP1A2、CYP2A6、CYP2B6、CYP2C8、CYP2C9、CYP2C19、CYP2D6、CYP2E1、CYP3A4和CYP3A5)在37°C下与2μmol/L安洛替尼温育60分钟。温育分两次进行,酶浓度为50 pmol P450/mL。在3000×g下离心10分钟后,通过液相色谱/质谱分析所得上清液。 为了比较P450介导的安洛替尼氧化的种间差异,在NADPH存在的情况下,将终浓度为2μmol/L的化合物与大鼠肝微粒体、犬肝微粒体制备和人肝微粒体孵育。在37°C下进行两次孵育3、7.5、15、30或60分钟。孵育条件与Hu等人所述的相同11。种间差异在代谢稳定性和代谢物形成方面具有特征。 安洛替尼对人药物代谢酶和人药物转运蛋白活性抑制作用的体外评估[3] 使用cDNA表达的CYP1A2、CYP2B6、CYP2C8、CYP2C9、CYP2C19、CYP2D6、CYP3A4和CYP3A5评估安洛替尼抑制人类P450酶活性的潜力。3-氰基-7-乙氧基香豆素、7-乙氧基-4-三氟甲基香豆素、二苄基荧光素、7-甲氧基-4-三氟甲基香豆素、3-[2-(N,N-二乙基-N-甲基氨基)乙基]-7-甲氧基-4-甲基香豆素、7-苄氧基-三氟甲基香豆素和咪达唑仑分别用作CYP1A2/CYP2C19、CYP2B6、CYP2C8、CYP2C9、CYP2D6、CYP3A4和CYP3A5的探针底物,这些探针底物在孵育混合物中的浓度分别为5.0/25.0、2.5、1.0、75.0、1.5、50.0和2.0μmol/L。最初,在孵育混合物中以100μmol/L的浓度对安洛替尼对P450酶活性的抑制作用进行了三次评估。当表现出>50%的抑制作用时,确定了安洛替尼对P450酶的半最大抑制浓度(IC50)。每种孵育混合物由安洛替尼、cDNA表达的P450酶和相关的探针底物组成。阴性对照混合物含有0.2%甲醇代替安洛替尼,而阳性对照混合物含有阳性抑制剂代替安洛替尼。CYP1A2、CYP2B6、CYP2C8、CYP2C9、CYP2C19、CYP2D6和CYP3A4/CYP3A5的阳性对照抑制剂分别为呋喃茶碱、反苯环丙胺、槲皮素、磺胺苯唑、反苯环丙丙胺、奎尼丁和酮康唑。在通过加入NADPH生成系统开始反应之前,将孵育混合物平衡10分钟,该系统包含3.3 mmol/L氯化镁、3.3 mmol/L葡萄糖-6-磷酸、0.5 U/mL葡萄糖-6-磷酸脱氢酶和1.3 mmol/L NADP。CYP3A5-、CYP1A2-、CYP2B6-/CYP2C19-/CYP2D6-/CYP3A4-、CYP2C8-和CYP2C9介导的反应的孵育时间分别为10、15、30、40和45分钟。用等体积的冰冷乙腈终止酶促反应,但CYP2C8除外,使用等体积的2 mol/L氢氧化钠溶液终止反应,然后在37°C下孵育2小时。使用SpectraMax M2酶标仪(Molecular Devices,Sunnyvale,CA,USA)在选定的激发/发射波长下测量探针底物的荧光代谢物,即410/460、409/530、485/538和390/460 nm/nm,用于3-氰基-7-羟基香豆素、7-羟基-4-三氟甲基香豆素、荧光素和7-羟基香豆素/3-[2-(N,N-二乙氨基)乙基]-7-羟基-4-甲基香豆素,分别是CYP1A2/CYP2C19、CYP2B6/CYP2C9/CYP3A4、CYP2C8和CYP2D6探针底物的代谢产物。基于液相色谱/质谱的方法用于分析CYP3A5探针底物咪达唑仑的代谢产物1'-羟基咪达唑拉姆。 使用cDNA表达的UGT1A1、UGT1A3、UGT1A4、UGT1A6、UGT1A9、UGT2B7和UGT2B15评估安洛替尼抑制人UGT酶活性的潜力。β-雌二醇、4-甲基伞形酮、三氟拉嗪和senkyunolide I分别用作UGT1A1、UGT1A3/UGT1A6/UGT1A9/UGT2B7、UGT1A4和UGT2B15的探针底物,这些探针底物在培养混合物中的浓度分别为20、1000/100/10/300、40和20μmol/L。最初,在孵育混合物中100μmol/L的安洛替尼对UGT酶活性的抑制作用进行了三次评估。当显示>50%的抑制作用时,确定了安洛替尼对UGT酶的半最大抑制浓度(IC50)。每种孵育混合物均由安洛替尼、cDNA表达的UGT酶、甲卡西星和相关探针底物组成。阴性对照混合物含有0.2%甲醇代替安洛替尼,而阳性对照混合物含有阳性抑制剂代替安洛替尼。UGT1A1/UGT1A9、UGT1A3、UGT1A4、UGT1A6和UGT2B7/UGT2B15的阳性对照抑制剂分别为硝氟酸、阿扎那韦、hecogenin、苯丁酮和双氯芬酸。在加入UDPGA引发反应之前,将孵育混合物平衡5分钟。UGT1A1-/UGT1A6-、UGT1A3-、UGT1A4-、UGT139-、UGT2B7-和UGT2B15介导的酶反应的孵育时间分别为30、75、20、15、120和20分钟。通过加入两倍体积的冰冷甲醇,然后在1000×g下离心10分钟来停止这些反应。通过液相色谱/质谱分析上清液,以确定形成的葡糖苷酸的量,即UGT1A1、UGT1A3/UGT1A6/UGT1A9/UGT2B7、UGT1A4和UGT2B15的E3G、4-MUG、TFPG和senkyunolide I-7-O-β-葡糖苷酸。 为了评估安洛替尼抑制人类转运蛋白活性的潜力,如前所述,使用Lipofectamine 2000转染试剂(Invitrogen,Carlsbad,CA,USA)将人类有机阴离子转运多肽(OATP)1B1、OATP1B3、人类有机阳离子转运蛋白(OCT)2、人类有机阴离子转运体(OAT)1和OAT3质粒以及空载体分别引入HEK-293细胞12,13。最初,评估了100μmol/L的安洛替尼在孵育混合物中对OATP1B1、OATP1B3、OCT2、OAT1和OAT3活性的抑制作用,使用的探针底物分别是雌二醇-17β-D-葡糖苷酸、对氨基马尿酸、四乙基铵和雌酮-3-硫酸酯,所有孵育混合物的浓度均为10μmol/L。当显示抑制率>50%时,测定安洛替尼对转运蛋白的IC50。所有实验均进行了三次。通过液相色谱/质谱法测定孵育后细胞中探针底物的浓度。 使用表达转运蛋白的膜囊泡评估安洛替尼对人MDR1、MRP1和BCRP活性的抑制作用,并分别使用培养混合物中10μmol/L的人参皂苷Rg1、雌二醇-17β-D-葡糖苷酸和甲氨蝶呤作为探针底物。最初,评估了100μmol/L的安洛替尼在孵育混合物中对转乘器活性的抑制作用。当显示抑制率>50%时,测定安洛替尼对转运蛋白的IC50。Jiang等人12描述了使用探针底物的囊泡运输方法的细节,所有实验都进行了三次。通过液相色谱/质谱法测定孵育后囊泡内捕获的探针底物的数量。 检测盐酸安罗替尼对VEGFR2激酶的抑制活性:将药物与VEGFR2酪氨酸激酶的ATP结合口袋结合,通过检测激酶活性确定抑制效力,结果显示其对VEGFR2的IC50<1 nmol/L。生长因子刺激的受体磷酸化实验:将血清饥饿的HUVEC用不同浓度药物处理1.5h,再用20ng/mL VEGF刺激10min,裂解细胞后通过免疫印迹检测受体磷酸化水平 [1] 评估人CYPs对盐酸安罗替尼的代谢能力:将重组人CYP酶(50pmol P450/mL)与2μmol/L安罗替尼孵育60min,定量代谢产物以确定主要代谢酶(CYP3A代谢能力最强)。检测药物对药物代谢酶的抑制作用:将安罗替尼与人CYPs、UGTs和转运体共孵育,测定酶活性以确定IC50(对CYP3A4和CYP2C9的IC50<1μmol/L) [3] |
| 细胞实验 |
将细胞接种在96孔板中,并用一系列稀释的药物处理。孵育72小时后,通过硫罗丹明B(SRB)分析评估细胞增殖。30药物抑制细胞增殖的效力表示为IC50值,使用GraphPad Prism版本5曲线拟合软件测定[1]。
将不同浓度的测试剂应用于血清饥饿的 HUVEC、Mo7e、U-87MG 和 A431 细胞 1.5 小时。然后用 VEGF (20 ng/mL)、SCF-1 (2.5 ng/mL)、PDGF-BB (10 ng/mL) 或 EGF (10 ng/mL) 刺激细胞 10 分钟。指定的抗体用于探测细胞裂解物。 1. HUVEC增殖实验:将HUVEC与不同浓度的盐酸安罗替尼及20% FBS/20ng/mL VEGF共孵育,采用磺酰罗丹明B(SRB)法检测细胞活力,评估药物对VEGF/FBS刺激的增殖抑制作用 [1] 2. 肿瘤细胞增殖实验:肿瘤细胞在含10% FBS的培养基中培养并加入盐酸安罗替尼,通过SRB法检测细胞活力,确定药物对肿瘤细胞的直接抑制作用(需微摩尔浓度) [1] 3. HUVEC迁移和管形成实验:用盐酸安罗替尼处理HUVEC,观察并定量VEGF诱导的细胞迁移和FBS刺激的管形成情况,评估药物的抗血管生成活性 [1] 4. 膜通透性实验:在Caco-2细胞中检测盐酸安罗替尼的膜通透性,结果显示其具有良好的通透性特征 [3] 5. 转运体抑制实验:利用HEK293细胞评估盐酸安罗替尼对转运体的抑制作用,发现除对特定转运体有轻微影响外,整体抑制作用有限 [3] |
| 动物实验 |
human colon cancer SW620 xenograft model(Balb/cA-nude mice, 5-6 weeks old)
0.75, 1.5, 3 and 6 mg/kg oral Female nude mice (Balb/cA‐nude, 5‐6 weeks old), purchased from Shanghai Laboratory Animal Center (Chinese Academy of Sciences, Shanghai, China), were housed in sterile cages under laminar airflow hoods in a specific pathogen‐free room with a 12‐hour light/12‐hour dark schedule, and fed autoclaved chow and water ad libitum. Human tumor xenografts were established by s.c. inoculating cells into the left axilla of nude mice. When tumor volumes reached 100‐200 mm3, mice were divided randomly into control and treatment groups. Control groups were given vehicle alone, and treatment groups received oral anlotinib or sunitinib daily. Tumor volume was calculated as (length × width2)/2. Tumor growth inhibition was calculated from the start of treatment by comparing changes in tumor volumes for control and treatment groups.[1] Rat studies[3] Rats were randomly assigned to four groups (five male and five female rats per group) to receive a single oral dose of anlotinib at 1.5, 3, or 6 mg/kg (via gavage) or a single intravenous dose at 1.5 mg/kg (from the tail vein). Serial blood samples (around 0.25 mL; before and 5, 15, and 30 min and 1, 2, 4, 6, 8, 11, and 24 h after dosing) were collected in heparinized tubes from the orbital sinuses of rats under isoflurane anesthesia and centrifuged at 1300×g for 10 min to yield plasma fractions. Rats under isoflurane anesthesia were killed by bleeding from the abdominal aorta at 1, 4, 8, and 24 h (three male and three female rats per time point) after a single oral dose of anlotinib at 3 mg/kg. [3] Tumor-bearing mouse studies[3] Female tumor-bearing mice were randomly assigned to three groups (20 mice per group) to receive a single oral dose of anlotinib at 0.75, 1.5, or 3 mg/kg (via gavage). Mice under isoflurane anesthesia were killed by bleeding from the orbital sinus at 2, 4, 8, and 24 h (five mice per time point) after dosing. Dog study[3] Dogs were randomly assigned to four groups (three male and three female dogs per group) to receive a single oral dose of anlotinib at 0.5, 1, or 2 mg/kg (via gavage) or a single intravenous dose at 0.5 mg/kg (from left forelimb vein). 1. Xenograft tumor model in nude mice: Nude mice bearing SW620, U-87 MG, Caki-1, SK-OV-3, or Calu-3 tumor xenografts were orally administered Anlotinib Dihydrochloride or sunitinib once daily. For SW620 models, dosing continued for 18 days; for SK-OV-3 and Calu-3 models, dosing lasted 9 days. Tumor volume and mouse body weight were measured twice weekly, and CD31 immunohistochemistry was used to detect vascular density in tumor tissues [1] 2. Rat aortic ring assay: Explants of rat aorta were cultured in vitro and treated with Anlotinib Dihydrochloride; microvessel sprouting from the explants was observed and quantified to evaluate the inhibitory effect on angiogenesis [1] 3. Pharmacokinetic study in experimental animals: Rats and dogs were administered Anlotinib Dihydrochloride via oral or intravenous routes. Blood samples were collected at different time points, and plasma concentrations of the drug were measured by liquid chromatography/mass spectrometry to determine pharmacokinetic parameters (bioavailability, half-life, clearance). Tumor-bearing mice were given oral doses of the drug, and tissue homogenates were prepared to measure tissue concentrations [3] |
| 药代性质 (ADME/PK) |
Plasma pharmacokinetics of anlotinib in rats and in dogs [3]
\nMean plasma concentrations of anlotinib over time after a single dose of anlotinib in rats and dogs are shown in Figure 1; the plasma pharmacokinetic parameters of anlotinib are summarized in Table 1. After oral administration, levels of systemic exposure to anlotinib, i.e., plasma maximum concentration (Cmax) and area under the plasma concentration-time curve up to 24 h (AUC0-24 h), in female rats tended to be greater than those in male rats at the tested dose range 1.5–6 mg/kg, while gender differences in plasma Cmax and AUC0-24 h of anlotinib were not significant in dogs at the dose range 0.5–2 mg/kg. The plasma Cmax and AUC0-24h increased as the anlotinib dose increased in an over-proportional manner in rats and dogs (Table 2). Anlotinib was highly bound in rat, dog, and human plasma with unbound fractions in plasma (fu) of 2.9%, 4.0%, and 7.3%, respectively. These fu values were independent of total plasma concentration of anlotinib, suggesting that such total concentration of anlotinib is a good measure of the changes in its unbound concentration in plasma for the species. As shown in Table 3, anlotinib exhibited binding affinity for α1-acid glycoprotein similar to imatinib, another tyrosine kinase inhibitor. However, it exhibited substantially higher affinity for albumin than imatinib. Unlike imatinib with an nKα1-acid-glycoprotein/nKalbumin ratio of 92.0, such a ratio for anlotinib was 0.9 (<7.7), suggesting the high excess of plasma concentration of albumin (600 μmol/L) over α1-acid glycoprotein (20 μmol/L) could not be compensated for circulating anlotinib in humans. It is worth mentioning that anlotinib exhibited notably higher affinity for plasma lipoproteins, particularly for low density lipoproteins and very low density lipoproteins, than for albumin and α1-acid glycoprotein. After oral administration, anlotinib was rapidly absorbed from the gastrointestinal tract in rats and dogs (Figure 1). Dogs tended to exhibit greater oral bioavailability (F) of anlotinib than rats. Terminal half-lives (t1/2) of anlotinib in rats and dogs after intravenous administration were comparable with the respective t1/2 values after oral administration. Dogs exhibited a longer mean t1/2 of anlotinib than rats. This t1/2 difference appeared to be attributed mainly to interspecies difference in total plasma clearance (CLtot,p). Anlotinib's mean apparent volume of distribution at steady state (VSS) in rats was 40 times as much as the rat volume of total body water and the value of VSS in dogs was 12 times as much as the dog volume of total body water15, suggesting the compound was distributed widely into various body fluids and tissues. In rats that received an intravenous dose of anlonitib, only small amounts of the unchanged compound were excreted into urine, bile, and feces (Table 1), suggesting that metabolism was the major elimination route of anlotinib.\n \n\nIntestinal absorption-related properties of anlotinib [3] \nIntestinal absorption of a drug is a combined result of its solubility in gastrointestinal fluids, membrane permeability, and substrate specificity to efflux system of the intestinal epithelia. Anlotinib exhibited pH-dependent aqueous solubility, ie, >1 g/mL at pH 1.7 (the stomach), 114 μg/mL at pH 4.6 (the duodenum), and 0.89 μg/mL at pH 6.5 (the jejunum and the ileum). The solubility values of anlotinib at pH 1.7 and 4.6 were greater than the compound's minimum solubility necessary to achieve adequate intestinal absorption at the dose 6 mg/kg, but the solubility value at pH 6.5 was lower than the minimum solubility. The minimum solubility was deduced according to a bar chart, by Lipinski, that depicts the minimum solubility for compounds with low, medium, and high permeability at doses of 0.1, 1, and 10 mg/kg16. Anlotinib exhibited good membrane permeability across Caco-2 cell monolayers, expressing MDR1, MRP2, and BCRP, with a mean apparent permeability coefficient (Papp) of 3.5×10−6 cm/s. The compound exhibited a mean efflux ratio (EfR) of 0.91±0.22, suggesting that its transport across the cell monolayer did not affected by the apical efflux transporters in Caco-2 cells (Supplementary Figure S1). Physicochemical properties of anlotinib (predicted using ACD/Percepta; Toronto, Ontario, Canada), ie, molecular mass (407 Da; favorable value, <500 Da), hydrogen-bonding capacity (HBA+HBD, 6+3; <12), topological polar surface area (TPSA, 82.4 Å2; <140 Å2), and molecular flexibility (NROTB, 6; <10), supported its good membrane permeability. Measured LogD values were −0.89 at pH 1.7, 2.10 at pH 4.6, and 2.38 at pH 6.5 (favorable range, 0–5).\n \n\nTissue distribution of anlotinib in rats and tumor-bearing mice [3] \nLevels of various tissue exposures to anlotinib (measured using associated tissue homogenate samples) in rats and tumor-bearing mice after an oral dose of the compound were significantly higher than the associated systemic exposure level (Figure 2). In rats, the lung exhibited the highest exposure level, which was 197 times as high as the systemic exposure level. Meanwhile, the rat liver, kidneys, and heart also exhibited high exposure levels, which were 49, 54, and 32 times as much as the systemic exposure level. Anlotinib penetrated the rat brain, with a brain homogenate AUC0-24h level comparable to the associated plasma level. In tumor-bearing mice, the level of tumor tissue exposure to the compound increased as the dose increased; it was 13 times as high as the systemic exposure level.\n \n\nMetabolism of anlotinib [3] \nBecause neither hepatobiliary nor renal excretion of unchanged compound was the main elimination route, metabolites of anlotinib in rat and dog samples were detected and characterized. As a result, a total of 12 anlotinib metabolites (M1–M12) were detected in the plasma, bile, urine, and feces samples of rats after dosing (Table 4). Eight of the metabolites, ie, M2, M4, M5, M6, M8, M9, M10, and M11, were found in plasma. All these metabolites occurred in rat bile and urine samples, except for M7 and M11, which occurred only in rat bile samples. In dogs, a total of 5 metabolites of anlotinib were detected in plasma samples; they were M4, M8, M9, M10, and M11. After liquid chromatography/mass spectrometry-based characterization of these metabolites, metabolic pathways of anlotinib were proposed (Figure 3). The major metabolic pathways of anlotinib in rats were probably the hydroxylation to form M10 and M11 and the dealkylation to form M8. The metabolites M10 and M11 were two major plasma metabolites of anlotinib in rats, while M8 was further glucuronized to form M6, a major plasma and biliary metabolite of anlotinib. To further characterize these metabolic pathways, in vitro metabolism studies were performed for anlotinib. As a result, multiple human cytochrome P450 enzymes, ie, CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4, and CYP3A5, were found to be able to mediate the oxidation of anlotinib to form M10, M11, and M8 (Figure 4A). Among these enzymes were CYP3A4 and CYP3A5 that exhibited the greatest metabolic capabilities. As shown in Figure 4B, the metabolites M10, M11, and M8 were also detected in samples of anlotinib after being incubated with NADPH-fortified rat liver microsomes, dog liver microsomes, and human liver microsomes under the same conditions. The total amounts of metabolites formed were different for the tested liver microsomes of different species, suggesting the highest oxidation rate by rat liver microsomes followed by dog liver microsomes, and then by human liver microsomes.\n \n\nIn vitro inhibitory activity of anlotinib on drug metabolism enzymes and transporters [3] \nAs shown in Table 5, anlotinib exhibited, in vitro, significant potency to inhibit CYP3A4 and CYP2C9 with IC50 values of <1 μmol/L; such inhibitory potency towards CYP2C19, CYP2C8, UGT1A1, UGT1A4, UGT1A9, and UGT2B15 was moderate, with IC50 values of 1–10 μmol/L. This tyrosine kinase inhibitor exhibited low inhibitory potency in vitro towards human CYP2B6, CYP2D6, UGT1A6, UGT2B7, OATP1B1, OAT3, OCT2, MDR1, and BCRP with values of IC50 greater than 10 μmol/L. No significant inhibitory potency of anlotinib was found towards human CYP1A2, CYP3A5, OATP1B3, OAT1, and MRP1. Anlotinib was not an in vitro substrate of OATP1B1, OATP1B3, OAT1, OAT3, OCT2, MDR1, and BCRP (Supplementary Table S1). Anlotinib Dihydrochloride had long elimination half-lives and significant accumulation during multiple oral doses in humans. The 2/1 schedule with 12 mg once daily was identified as the maximum tolerated dose [2] Anlotinib Dihydrochloride was rapidly absorbed with good membrane permeability. Its oral bioavailability was 28%-58% in rats and 41%-77% in dogs. The terminal half-life was 5.1 ± 1.6 h in rats and 22.8 ± 11.0 h in dogs, with total plasma clearance of 5.35 ± 1.31 L·h⁻¹·kg⁻¹ in rats and 0.40 ± 0.06 L·h⁻¹/kg in dogs. It had a large apparent volume of distribution (27.6 ± 3.1 L/kg in rats, 6.6 ± 2.5 L/kg in dogs). Cytochrome P450-mediated metabolism was the major elimination pathway, with human CYP3A showing the greatest metabolic capability [3] |
| 毒性/毒理 (Toxicokinetics/TK) |
On the 4/0 schedule, no DLT was observed in the first four patients at the starting dose of 5 mg/day. However, at 10 mg/day, one patient developed grade 3 hypertension among the first three patients treated. An additional patient was enrolled and also developed grade 3 hypertension. Therefore, the further dose escalation was halted. Meanwhile, PK study revealed a continuously significant anlotinib accumulation in patients who received continuous administration (data not shown). Based on the PK profile of anlotinib and the two DLTs observed at the dose of 10 mg/day, we modified the administration protocol from the 4/0 schedule to the 2/1.[2]
On the 2/1 schedule, because none of the three patients experienced DLT at initial doses of 10 mg/day, the dose escalation proceeded to 16 mg/day. Two of the three patients in the 16 mg cohort experienced DLT (one grade 3 fatigue and one grade 3 hypertension). Therefore, the MTD had been exceeded, and the next lower dose of 12 mg/day was further evaluated by entering additional patients. None of the initial three patients experienced grade 3/4 adverse events. On the basis, 12 mg once daily was selected for the expanding study. [2] A total of 21 patients received the 12 mg/day dose on the 2/1 schedule. During the first 2 cycles, all the patients experienced an adverse event of any causality. All the hematologic toxicities were mild. As illustrated in Table 2, the most common non-hematologic adverse events were hypothyroidism, triglyceride elevation, total cholesterol elevation, ALT elevation, diarrhea, and proteinuria. During the first 2 cycles, a total of two patients (10 %) experienced grade 3 adverse events (one triglyceride elevation and one lipase elevation). During all treatment cycles, there were six patients (29 %) with grade 3/4 adverse events. The most common (>5 %) non-hematologic grade 3 adverse events were hypertension, triglyceride elevation, hand-foot skin reaction, and lipase elevation. On the 4-week consecutive (4/0) schedule, dose-limiting toxicity (DLT) of Anlotinib Dihydrochloride was grade 3 hypertension at 10 mg. On the 2/1 schedule, DLT was grade 3 hypertension and grade 3 fatigue at 16 mg. The main serious adverse effects in patients were hypertension, triglyceride elevation, hand-foot skin reaction, and lipase elevation [2] Anlotinib Dihydrochloride was highly bound to plasma proteins (97% in rats, 96% in dogs, 93% in humans), predominantly binding to albumin and lipoproteins in human plasma (not to α₁-acid glycoprotein or γ-globulins). It exhibited limited in vitro potency to inhibit most human CYPs, UGTs, and transporters, except for CYP3A4 and CYP2C9 (IC50 < 1 μmol/L). Drug interaction indices were 0.16 for CYP3A4 and 0.02 for CYP2C9, indicating a low propensity for drug-drug interactions [3] |
| 参考文献 | |
| 其他信息 |
Catequentinib Hydrochloride is the hydrochloride salt form of catequentinib, a receptor tyrosine kinase (RTK) inhibitor with potential antineoplastic and anti-angiogenic activities. Upon administration, catequentinib targets multiple RTKs, including vascular endothelial growth factor receptor type 2 (VEGFR2) and type 3 (VEGFR3). This agent may both inhibit angiogenesis and halt tumor cell growth.
\nbrogating tumor angiogenesis by inhibiting vascular endothelial growth factor receptor-2 (VEGFR2) has been established as a therapeutic strategy for treating cancer. However, because of their low selectivity, most small molecule inhibitors of VEGFR2 tyrosine kinase show unexpected adverse effects and limited anticancer efficacy. In the present study, we detailed the pharmacological properties of anlotinib, a highly potent and selective VEGFR2 inhibitor, in preclinical models. Anlotinib occupied the ATP-binding pocket of VEGFR2 tyrosine kinase and showed high selectivity and inhibitory potency (IC50 <1 nmol/L) for VEGFR2 relative to other tyrosine kinases. Concordant with this activity, anlotinib inhibited VEGF-induced signaling and cell proliferation in HUVEC with picomolar IC50 values. However, micromolar concentrations of anlotinib were required to inhibit tumor cell proliferation directly in vitro. Anlotinib significantly inhibited HUVEC migration and tube formation; it also inhibited microvessel growth from explants of rat aorta in vitro and decreased vascular density in tumor tissue in vivo. Compared with the well-known tyrosine kinase inhibitor sunitinib, once-daily oral dose of anlotinib showed broader and stronger in vivo antitumor efficacy and, in some models, caused tumor regression in nude mice. Collectively, these results indicate that anlotinib is a well-tolerated, orally active VEGFR2 inhibitor that targets angiogenesis in tumor growth, and support ongoing clinical evaluation of anlotinib for a variety of malignancies.[1] \nBackground: Anlotinib is a novel multi-target tyrosine kinase inhibitor that is designed to primarily inhibit VEGFR2/3, FGFR1-4, PDGFR α/β, c-Kit, and Ret. We aimed to evaluate the safety, pharmacokinetics, and antitumor activity of anlotinib in patients with advanced refractory solid tumors.\n\nMethods: Anlotinib (5-16 mg) was orally administered in patients with solid tumor once a day on two schedules: (1) four consecutive weeks (4/0) or (2) 2-week on/1-week off (2/1). Pharmacokinetic sampling was performed in all patients. Twenty-one patients were further enrolled in an expanded cohort study on the recommended dose and schedule. Preliminary tumor response was also assessed.\n\nResults: On the 4/0 schedule, dose-limiting toxicity (DLT) was grade 3 hypertension at 10 mg. On the 2/1 schedule, DLT was grade 3 hypertension and grade 3 fatigue at 16 mg. Pharmacokinetic assessment indicated that anlotinib had long elimination half-lives and significant accumulation during multiple oral doses. The 2/1 schedule was selected, with 12 mg once daily as the maximum tolerated dose for the expanding study. Twenty of the 21 patients (with colon adenocarcinoma, non-small cell lung cancer, renal clear cell cancer, medullary thyroid carcinoma, and soft tissue sarcoma) were assessable for antitumor activity of anlotinib: 3 patients had partial response, 14 patients had stable disease including 12 tumor burden shrinkage, and 3 had disease progression. The main serious adverse effects were hypertension, triglyceride elevation, hand-foot skin reaction, and lipase elevation.\n\nConclusions: At the dose of 12 mg once daily at the 2/1 schedule, anlotinib displayed manageable toxicity, long circulation, and broad-spectrum antitumor potential, justifying the conduct of further studies.[2] \n\nAnlotinib is a new oral tyrosine kinase inhibitor; this study was designed to characterize its pharmacokinetics and disposition. Anlotinib was evaluated in rats, tumor-bearing mice, and dogs and also assessed in vitro to characterize its pharmacokinetics and disposition and drug interaction potential. Samples were analyzed by liquid chromatography/mass spectrometry. Anlotinib, having good membrane permeability, was rapidly absorbed with oral bioavailability of 28%-58% in rats and 41%-77% in dogs. Terminal half-life of anlotinib in dogs (22.8±11.0 h) was longer than that in rats (5.1±1.6 h). This difference appeared to be mainly associated with an interspecies difference in total plasma clearance (rats, 5.35±1.31 L·h-1·kg-1; dogs, 0.40±0.06 L·h-1/kg-1). Cytochrome P450-mediated metabolism was probably the major elimination pathway. Human CYP3A had the greatest metabolic capability with other human P450s playing minor roles. Anlotinib exhibited large apparent volumes of distribution in rats (27.6±3.1 L/kg) and dogs (6.6±2.5 L/kg) and was highly bound in rat (97%), dog (96%), and human plasma (93%). In human plasma, anlotinib was predominantly bound to albumin and lipoproteins, rather than to α1-acid glycoprotein or γ-globulins. Concentrations of anlotinib in various tissue homogenates of rat and in those of tumor-bearing mouse were significantly higher than the associated plasma concentrations. Anlotinib exhibited limited in vitro potency to inhibit many human P450s, UDP-glucuronosyltransferases, and transporters, except for CYP3A4 and CYP2C9 (in vitro half maximum inhibitory concentrations, <1 μmol/L). Based on early reported human pharmacokinetics, drug interaction indices were 0.16 for CYP3A4 and 0.02 for CYP2C9, suggesting that anlotinib had a low propensity to precipitate drug interactions on these enzymes. Anlotinib exhibits many pharmacokinetic characteristics similar to other tyrosine kinase inhibitors, except for terminal half-life, interactions with drug metabolizing enzymes and transporters, and plasma protein binding.[3] Anlotinib Dihydrochloride is a highly potent and selective VEGFR2 inhibitor that targets tumor angiogenesis. It is a well-tolerated, orally active agent with stronger and broader in vivo antitumor efficacy than sunitinib in preclinical models, supporting clinical evaluation for various malignancies [1] Anlotinib Dihydrochloride is a novel oral multi-target tyrosine kinase inhibitor. At the dose of 12 mg once daily on the 2/1 schedule, it displayed manageable toxicity, long circulation, and broad-spectrum antitumor potential in patients with advanced refractory solid tumors [2] Anlotinib Dihydrochloride has pharmacokinetic characteristics similar to other tyrosine kinase inhibitors, with differences in terminal half-life, interactions with drug-metabolizing enzymes/transporters, and plasma protein binding. It has a low propensity to precipitate drug interactions despite inhibiting CYP3A4 and CYP2C9 in vitro [3] |
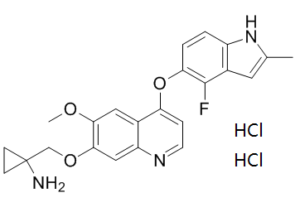
| 分子式 |
C23H24CL2FN3O3
|
|---|---|
| 分子量 |
480.36
|
| 精确质量 |
479.117
|
| 元素分析 |
C, 57.51; H, 5.04; Cl, 14.76; F, 3.96; N, 8.75; O, 9.99
|
| CAS号 |
1360460-82-7
|
| 相关CAS号 |
1058156-90-3;1360460-82-7 (HCl);
|
| PubChem CID |
57380530
|
| 外观&性状 |
Solid powder
|
| tPSA |
82.4Ų
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
32
|
| 分子复杂度/Complexity |
606
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CC1=CC2=C(N1)C=CC(=C2F)OC3=C4C=C(C(=CC4=NC=C3)OCC5(CC5)N)OC.Cl.Cl
|
| InChi Key |
UUAKQNIPIXQZFN-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C23H22FN3O3.2ClH/c1-13-9-15-16(27-13)3-4-19(22(15)24)30-18-5-8-26-17-11-21(20(28-2)10-14(17)18)29-12-23(25)6-7-23;;/h3-5,8-11,27H,6-7,12,25H2,1-2H3;2*1H
|
| 化学名 |
1-[[4-[(4-fluoro-2-methyl-1H-indol-5-yl)oxy]-6-methoxyquinolin-7-yl]oxymethyl]cyclopropan-1-amine;dihydrochloride
|
| 别名 |
AL3818 dihydrochloride; AL-3818 dihydrochloride; AL 3818 dihydrochloride; Anlotinib HCl; AL3818; Anlotinib dihydrochloride; Anlotinib HCl; 1360460-82-7; Anlotinib hydrochloride; AL3818 dihydrochloride; Catequentinib Hydrochloride; CATEQUENTINIB DIHYDROCHLORIDE; A3749M6582;AL 3818; AL-3818; Anlotinib; Catequentinib
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO:≥ 60 mg/mL
Water: Ethanol: |
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0818 mL | 10.4089 mL | 20.8177 mL | |
| 5 mM | 0.4164 mL | 2.0818 mL | 4.1635 mL | |
| 10 mM | 0.2082 mL | 1.0409 mL | 2.0818 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05481645 | Recruiting | Drug: TQB2450 injection Drug: Carboplatin Injection |
Advanced Endometrial Cancer Sarcoma of Uterus |
Chia Tai Tianqing Pharmaceutical Group Co., Ltd. |
August 22, 2022 | Phase 2 |
The lung metastasis changes in patients of alveolar soft tissue sarcoma with lung metastasis during treatment.J Hematol Oncol.2016 Oct 4;9(1):105. |
Duration of treatment and tumor size changes of 20 patients who received 12mg QD at the 2/1 schedule. J Hematol Oncol.2016 Oct 4;9(1):105. |
Plasma concentrations of anlotinib over time after a single oral dose of anlotinib capsules at 5 (green line), 10 (purple line), 12 (blue line), or 16mg anlotinib/person (red line) in male (solid circles) and female cancer patients (open circles) (a).bCorrelation of dose with plasma AUC0–120h.cCorrelation of dose with plasmaCmax.dCorrelation of dose witht1/2.ePlasma concentrations of anlotinib (24h after daily dosing) over time during multiple oral doses of anlotinib capsules at 12mg anlotinib/person/day in female cancer patients.fPlasma concentrations of anlotinib (24h after daily dosing) over time during multiple oral doses of anlotinib capsules at 12mg anlotinib/person/day in male cancer patients.J Hematol Oncol.2016 Oct 4;9(1):105. |