| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500μg |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
VIP/vasoactive intestinal polypeptide; vasodilatory
|
|---|---|
| 体外研究 (In Vitro) |
Aviptadil是一种人类血管活性肠多肽(VIP)的合成形式,已被FDA授予治疗ARDS的孤儿药指定,并获准进入FDA冠状病毒技术加速计划。
VIP与肺泡II型(ATII)细胞上的VPAC1受体结合。ATII细胞仅占肺上皮细胞的5%,但对肺泡1型细胞的氧转移、表面活性剂的产生和维持至关重要。70%的VIP与这种受体结合。II型细胞也是SARS-CoV-2病毒通过ACE2表面受体选择性攻击的细胞。[1]
|
| 体内研究 (In Vivo) |
肺动脉高压(PH)导致右心室负荷增加、心力衰竭和死亡。在特发性肺动脉高压(PAH)中,血管舒张血管活性肠肽(aviptadil)缺乏。本研究的目的是测试慢性PH患者单剂量吸入阿维他地尔对血液动力学和血气的急性影响,以及安全性。共有20例PH患者(9例为PAH, 8例为肺部疾病,3例为慢性血栓栓塞性PH)在右心导管插入期间吸入单剂量100微克的阿维他地尔。测量血液动力学和血气。阿维他地尔气雾剂引起小而短暂但显著的选择性肺血管舒张,提高脑卒中容量和混合静脉氧饱和度。总的来说,6名患者的肺血管阻力降低了20%。在有明显肺部疾病的患者中,阿维他地尔倾向于改善氧合。阿维他地尔气雾剂的肺血管扩张作用是适度和短暂的,没有引起任何副作用,导致右心室负荷减少,而不影响全身血压。阿维他地尔吸入可改善明显肺部疾病患者的氧合。需要进一步的研究来评估阿维他地尔气雾剂的全部治疗潜力,包括更高的剂量和慢性治疗。[5]
|
| 动物实验 |
Acute Lung Injury, which triggers Critical COVID-19 is a known lethal complication of Corona Virus (SARS-CoV-2) infection. Conventional medical therapy, including intensive care and respiratory support is associated with an 80% mortality. Aviptadil, a synthetic form of Human Vasoactive Intestinal Polypeptide (VIP) has been awarded FDA Orphan Drug Designation for the treatment of ARDS and admitted to the FDA CoronaVirus Technology Accelerator Program.
VIP binds to VPAC1 receptors on the pulmonary Alveolar Type II (ATII) cell. ATII cells comprise only 5% of lung epithelial cells but are critical for oxygen transfer, surfactant production, and maintenance of Alveolar Type 1 cells. 70% of VIP binds to this receptor. The Type II cell is also the cell selectively attacked by the SARS-CoV-2 virus via the ACE2 surface receptor.
Nonclinical studies demonstrate that VIP is highly concentrated in the lung and specifically bound to the ATII cell, where it prevents NMDA-induced caspase-3 activation in the lung, inhibits IL6 and TNFa production, protects against HCl-induced pulmonary edema, and upregulates surfactant production, These and other effects have been observed in numerous animal model systems of lung injury in mice, rats, guinea pigs, sheep, swine, and dogs. In these models, Aviptadil restores barrier function at the endothelial/alveolar interface and thereby protects the lung and other organs from failure.
Aviptadil ihas a demonstrated 20 year history of safety in phase 2 trials for Sarcoid, Pulmonary Fibrosis, Bronchospasm, and a phase I trial in ARDS. In that phase I trial, 8 patients with severe ARDS on mechanical ventilation were treated with ascending doses of VIP. Seven of the 8 patients were successfully extubated and were alive at the five day timepoint. Six left the hospital and one died of an unrelated cardiac event.
Five phase 2 trials of aviptadil have been conducted under European regulatory authority. Numerous healthy volunteer studies have shown that i.v. infusion of Aviptadil is well tolerated with few adverse effects including alterations in blood pressure, heart rate, or ECG. In addition to published studies of human use, Aviptadil has been used on a compounded basis in certain ICUs for many years in the belief that it preserves life and restores function in pulmonary hypertension, ARDS, and Acute Lung Injury (ALI).
In this study, patients who are hospitalized for Critical COVID-19 infection with respiratory failure will be randomly allocated to Aviptadil administered by intravenous infusion in addition to maximal intensive care vs. maximal intensive care alone. Primary endpoints will be improvement in blood oxygenation and mortality. [1]
|
| 参考文献 | |
| 其他信息 |
Aviptadil is a synthetic form of vasoactive intestinal polypeptide (VIP), with potential anti-cytokine, anti-inflammatory, and immune-regulatory activities. Upon administration, aviptadil mimics endogenous VIP. In the lungs, aviptadil may prevent N-Methyl-D-aspartic acid (NMDA)-induced caspase-3 activation, inhibits the production of certain pro-inflammatory mediators, such as interleukin-6 (IL-6) and tumor-necrosis factor-alpha (TNFa), and may protect the lungs against a cytokine storm and inflammation. As cytokines cause the air sacs of the lungs to fill with water, making the sacs impermeable to oxygen, aviptadil may protect against pulmonary edema, and restores the barrier function at the endothelial/alveolar interface. This may improve blood oxygenation, respiratory distress, and prevent lung injury. VIP is a naturally synthesized peptide hormone that is highly concentrated in the lungs.
AVIPTADIL is a Protein drug with a maximum clinical trial phase of III (across all indications) and has 4 investigational indications. Biomedical advances over the last decade have identified the central role of proliferative pulmonary arterial smooth muscle cells (PASMCs) in the development of pulmonary hypertension (PH). Furthermore, promoters of proliferation and apoptosis resistance in PASMCs and endothelial cells, such as aberrant signal pathways involving growth factors, G protein-coupled receptors, kinases, and microRNAs, have also been described. As a result of these discoveries, PH is currently divided into subgroups based on the underlying pathology, which allows focused and targeted treatment of the condition. The defining features of PH, which subsequently lead to vascular wall remodeling, are dysregulated proliferation of PASMCs, local inflammation, and apoptosis-resistant endothelial cells. Efforts to assess the relative contributions of these factors have generated several promising targets. This review discusses recent novel targets of therapies for PH that have been developed as a result of these advances, which are now in pre-clinical and clinical trials (e.g., imatinib [phase III]; nilotinib, AT-877ER, rituximab, tacrolimus, paroxetine, sertraline, fluoxetine, bardoxolone methyl [phase II]; and sorafenib, FK506, aviptadil, endothelial progenitor cells (EPCs) [phase I]). While substantial progress has been made in recent years in targeting key molecular pathways, PH still remains without a cure, and these novel therapies provide an important conceptual framework of categorizing patients on the basis of molecular phenotype(s) for effective treatment of the disease.[2] Background: Pulmonary Arterial Hypertension (PAH) remains a therapeutic challenge, and the search continues for more effective drugs and drug combinations. We recently reported that deletion of the vasoactive intestinal peptide (VIP) gene caused the spontaneous expression of a PH phenotype that was fully corrected by VIP. The objectives of this investigation were to answer the questions: 1) Can VIP protect against PH in other experimental models? and 2) Does combining VIP with an endothelin (ET) receptor antagonist bosentan enhance its efficacy? Methods: Within 3 weeks of a single injection of monocrotaline (MCT, s.c.) in Sprague Dawley rats, PAH developed, manifested by pulmonary vascular remodeling, lung inflammation, RV hypertrophy, and death within the next 2 weeks. MCT-injected animals were either untreated, treated with bosentan (p.o.) alone, with VIP (i.p.) alone, or with both together. We selected this particular combination upon finding that VIP down-regulates endothelin receptor expression which is further suppressed by bosentan. Therapeutic outcomes were compared as to hemodynamics, pulmonary vascular pathology, and survival. Results: Treatment with VIP, every other day for 3 weeks, begun on the same day as MCT, almost totally prevented PAH pathology, and eliminated mortality for 45 days. Begun 3 weeks after MCT, however, VIP only partially reversed PAH pathology, though more effectively than bosentan. Combined therapy with both drugs fully reversed the pathology, while preventing mortality for at least 45 days. Conclusions: 1) VIP completely prevented and significantly reversed MCT-induced PAH; 2) VIP was more effective than bosentan, probably because it targets a wider range of pro-remodeling pathways; and 3) combination therapy with VIP plus bosentan was more effective than either drug alone, probably because both drugs synergistically suppressed ET-ET receptor pathway.[3] Background: Vasoactive intestinal peptide (VIP) has been reported to have some properties that provide protection from lung injury. Furthermore, its protective effect in cold storage of donor lungs has been confirmed. We examined its effect and the timing of administration in an in vivo rat lung transplantation model. Methods: All lungs were flushed with low-potassium dextran-1% glucose solution, and orthotopic left lung transplantations were performed. Rats were divided into four groups (n = 6). Group I received no preservation or storage. Groups II, III, and IV grafts were stored for 18 hours at 4 degrees C. Group II received no VIP. Group III received VIP (0.1 g/ml) via the flush solution. Group IV recipients received VIP (3 microg/kg) intravenously just after reperfusion. Twenty-four hours after transplantation, the right main pulmonary artery and right main bronchus were ligated, and the rats were ventilated with 100% O2 for 5 minutes. Mean pulmonary arterial pressure, peak airway pressure, blood gas analysis, serum lipid peroxide level, tissue myeloperoxidase activity, and wet-dry weight ratio were measured. Results: The partial O2 tension values of groups III and IV were better than group II (groups II, III, and IV: 147.4 +/- 71.4, 402.1 +/- 64.8, 373.4 +/- 81.0 mm Hg; p < 0.05). Peak airway pressure was lower in groups III and IV than in group II (groups II, III, and IV: 19.7 +/- 0.8, 16.7 +/- 0.9. and 16.3 +/- 1.0 mm Hg; p < 0.05). Mean pulmonary arterial pressure in group III was lower than group II (groups II and III: 36.3 +/- 3.0 and 22.1 +/- 2.2 mm Hg; p < 0.01). Wet-dry weight ratio in group III was lower than in groups II and IV (group II, III, and IV: 5.2 +/- 0.2, 4.4 +/- 0.2, and 5.2 +/- 0.3; II vs III; p < 0.05, III vs IV; p < 0.01). Serum lipid peroxide levels in groups III and IV were significantly lower (groups II, III, and IV: 2.643 +/- 0.913, 0.455 +/- 0.147, and 0.325 +/- 0.124 nmol/ml; p < 0.01). Conclusion: VIP ameliorates reperfusion injury in an in vivo rat lung transplantation model. Either administration of VIP via the flush solution or systemically just after reperfusion was associated with improved pulmonary function.[4] |
| 分子式 |
C147H238N44O42S
|
|---|---|
| 分子量 |
3325.80
|
| 精确质量 |
3323.756
|
| 元素分析 |
C, 52.19; H, 7.15; N, 16.89; O, 22.87; S, 0.90
|
| CAS号 |
40077-57-4
|
| 相关CAS号 |
Aviptadil acetate;1444827-29-5
|
| PubChem CID |
16132300
|
| 序列 |
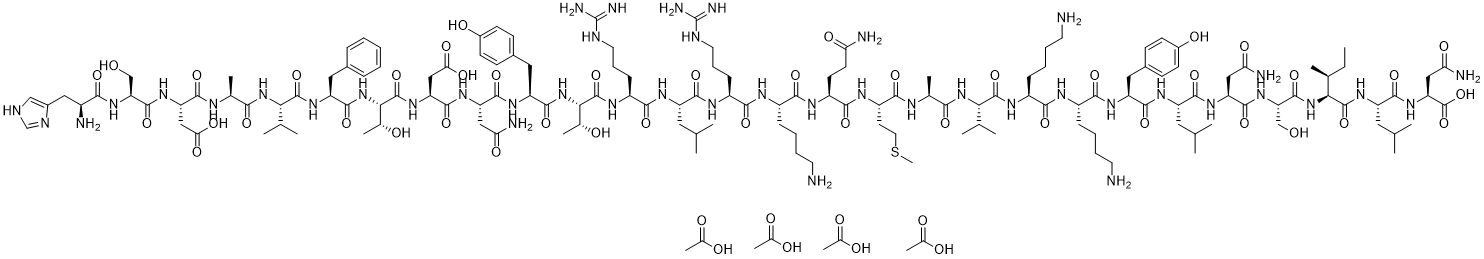
HSDAVFTDNYTRLRKQMAVKKYLNSILN-NH2
H-His-Ser-Asp-Ala-Val-Phe-Thr-Asp-Asn-Tyr-Thr-Arg-Leu-Arg-Lys-Gln-Met-Ala-Val-Lys-Lys-Tyr-Leu-Asn-Ser-Ile-Leu-Asn-OH |
| 短序列 |
HSDAVFTDNYTRLRKQMAVKKYLNSILN
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 折射率 |
1.660
|
| LogP |
-5.39
|
| tPSA |
1478.99
|
| SMILES |
CC[C@@H]([C@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](N)CC1=CN=CN1)=O)CO)=O)CC(O)=O)=O)C)=O)C(C)C)=O)CC2=CC=CC=C2)=O)[C@H](O)C)=O)CC(O)=O)=O)CC(N)=O)=O)CC3=CC=C(O)C=C3)=O)[C@H](O)C)=O)CCCNC(N)=N)=O)CC(C)C)=O)CCCNC(N)=N)=O)CCCCN)=O)CCC(N)=O)=O)CCSC)=O)C)=O)C(C)C)=O)CCCCN)=O)CCCCN)=O)CC4=CC=C(O)C=C4)=O)CC(C)C)=O)CC(N)=O)=O)CO)=O)C(N[C@H](C(N[C@H](C(N)=O)CC(N)=O)=O)CC(C)C)=O)C
|
| InChi Key |
CBTPBFFYMHBLFP-KQVGQEDNSA-N
|
| InChi Code |
InChI=1S/C147H237N43O43S.4C2H4O2/c1-18-75(12)115(142(229)180-96(56-72(6)7)131(218)183-104(145(232)233)63-110(155)200)188-139(226)106(68-192)185-134(221)101(62-109(154)199)177-130(217)95(55-71(4)5)174-132(219)97(58-81-37-41-84(195)42-38-81)175-125(212)88(33-23-26-49-149)167-123(210)89(34-24-27-50-150)171-140(227)113(73(8)9)186-118(205)76(13)164-121(208)93(47-53-234-17)170-127(214)92(45-46-107(152)197)169-122(209)87(32-22-25-48-148)166-124(211)90(35-28-51-161-146(156)157)168-129(216)94(54-70(2)3)173-126(213)91(36-29-52-162-147(158)159)172-143(230)116(78(15)193)189-136(223)98(59-82-39-43-85(196)44-40-82)176-133(220)100(61-108(153)198)178-135(222)103(65-112(203)204)182-144(231)117(79(16)194)190-137(224)99(57-80-30-20-19-21-31-80)181-141(228)114(74(10)11)187-119(206)77(14)165-128(215)102(64-111(201)202)179-138(225)105(67-191)184-120(207)86(151)60-83-66-160-69-163-83;4*1-2(3)4/h19-21,30-31,37-44,66,69-79,86-106,113-117,191-196H,18,22-29,32-36,45-65,67-68,148-151H2,1-17H3,(H2,152,197)(H2,153,198)(H2,154,199)(H2,155,200)(H,160,163)(H,164,208)(H,165,215)(H,166,211)(H,167,210)(H,168,216)(H,169,209)(H,170,214)(H,171,227)(H,172,230)(H,173,213)(H,174,219)(H,175,212)(H,176,220)(H,177,217)(H,178,222)(H,179,225)(H,180,229)(H,181,228)(H,182,231)(H,183,218)(H,184,207)(H,185,221)(H,186,205)(H,187,206)(H,188,226)(H,189,223)(H,190,224)(H,201,202)(H,203,204)(H,232,233)(H4,156,157,161)(H4,158,159,162);4*1H3,
|
| 化学名 |
H-His-Ser-Asp-Ala-Val-Phe-Thr-Asp-Asn-Tyr-Thr-Arg-Leu-Arg-Lys-Gln-Met-Ala-Val-Lys-Lys-Tyr-Leu-Asn-Ser-Ile-Leu-Asn-NH2 tetraacetic acid.
|
| 别名 |
Aviptadil Acetate; VIP; Invicorp; Aviptadil; 40077-57-4; (2S)-4-amino-2-[[(2S)-2-[[(2S,3S)-2-[[(2S)-2-[[(2S)-4-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-6-amino-2-[[(2S)-6-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-5-amino-2-[[(2S)-6-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S,3R)-2-[[(2S)-2-[[(2S)-4-amino-2-[[(2S)-2-[[(2S,3R)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-amino-3-(1H-imidazol-5-yl)propanoyl]amino]-3-hydroxypropanoyl]amino]-3-carboxypropanoyl]amino]propanoyl]amino]-3-methylbutanoyl]amino]-3-phenylpropanoyl]amino]-3-hydroxybutanoyl]amino]-3-carboxypropanoyl]amino]-4-oxobutanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-3-hydroxybutanoyl]amino]-5-carbamimidamidopentanoyl]amino]-4-methylpentanoyl]amino]-5-carbamimidamidopentanoyl]amino]hexanoyl]amino]-5-oxopentanoyl]amino]-4-methylsulfanylbutanoyl]amino]propanoyl]amino]-3-methylbutanoyl]amino]hexanoyl]amino]hexanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-4-methylpentanoyl]amino]
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ~100 mg/mL (~30.07 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.3007 mL | 1.5034 mL | 3.0068 mL | |
| 5 mM | 0.0601 mL | 0.3007 mL | 0.6014 mL | |
| 10 mM | 0.0301 mL | 0.1503 mL | 0.3007 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。