| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| Other Sizes |
|
| 靶点 |
5-HT1A Receptor
|
|---|---|
| 体外研究 (In Vitro) |
F13640(贝维拉多)是一种新型5-HT(1A)受体激动剂,与其他受体和结合位点相比具有特殊的选择性[1]。F13640以相似的效力激活前额叶皮层中的5-HT(1A)自身受体和突触后5-HT(1A)受体。这两种活性都可能与化合物的镇痛特性有关。[1]
|
| 体内研究 (In Vivo) |
F13640在0.2-18.2μg kg(-1)静脉注射时降低了中缝背侧5-羟色胺能神经元的活性(累积剂量;ED(50)=0.69μg kg。随后给予5-HT(1A)受体拮抗剂(±)WAY100635可逆转这两种作用。在微透析研究中,F13640(0.04-0.63 mg kg(-1),i.p.)剂量依赖性地降低了海马和mPFC中的细胞外5-HT。同样,F13640(0.01-2.5 mg kg(-1),i.p.)剂量依赖性地增加mPFC中的细胞外DA,这种作用依赖于mPFC中突触后5-HT(1A)受体的激活。mPFC(1-1000μM)中F13640的局部灌注也以浓度依赖的方式增加了细胞外DA。预先(±)WAY100635给药可预防F13640的全身和局部影响[1]。
|
| 动物实验 |
Rats were anaesthetized with chloral hydrate (400–500 mg kg−1, i.p.) or isoflurane. A guide cannula with a dummy probe was stereotaxically implanted into the mPFC, stereotaxic coordinates: AP +3.0 mm, L +0.8 mm, DV −1.7 mm, or the hippocampus: AP −4.8 mm, L +4.6 mm, DV −4.6 mm, from bregma and skull surface. Following surgery and recovery from anesthesia, animals were returned to their home cages. At the end of the day, each rat was placed in a microdialysis cage. On the following day, the dummy probe was replaced by a microdialysis probe (3 mm length, 0.5 mm diameter; CMA, Microdialysis AB). The probe was continuously perfused (1.1 μl min−1) with artificial CSF (aCSF) containing 1 μM citalopram for the measure of 5-HT. At least 2 h after probe insertion, samples were collected every 20 min with the first four samples used for baseline. For the experiment with systemic administration of the compounds, saline or (±)WAY100635 were injected s.c., followed, 40 min later, by i.p. administration of saline or F13640. For the experiments with local perfusion, saline was injected s.c. and 40 min later, F13640 was added to the perfusion medium for the concentration–response experiment. For the antagonism, (±)WAY100635 (or aCSF) was delivered through the dialysis probe and 40 min later, F13640 was added to the perfusion medium. Samples were collected for 140 min after administration or beginning of the perfusion of the agonist. At the end of the experiment, rats were killed by anesthetic overdose (pentobarbital 160 mg kg−1, i.p.) and the brain was removed, frozen and cut in a cryomicrotome (Jung Frigocut 2800) to verify the placement of the probe.[1]
|
| 药代性质 (ADME/PK) |
NLX-112 (i.e., F13640, befiradol) exhibits nanomolar affinity, exceptional selectivity and full agonist efficacy at serotonin 5-HT1A receptors. NLX-112 shows efficacy in rat, marmoset and macaque models of L-DOPA induced dyskinesia (LID) in Parkinson's disease and has shown clinical efficacy in a Phase 2a proof-of-concept study for this indication. Here we investigated, in rats, its pharmacodynamic, pharmacokinetic (PK) and brain 5-HT1A receptor occupancy profiles, and its PK properties in the absence and presence of L-DOPA. Total and free NLX-112 exposure in plasma, CSF and striatal ECF was dose-proportional over the range tested (0.04, 0.16 and 0.63 mg/kg i.p.). NLX-112 exposure increased rapidly (Tmax 0.25-0.5h) and exhibited approximately threefold longer half-life in brain than in plasma (1.1 and 3.6h, respectively). At a pharmacologically relevant dose of 0.16 mg/kg i.p., previously shown to elicit anti-LID activity in parkinsonian rats, brain concentration of NLX-112 was 51-63 ng/g from 0.15 to 1h. In microPET imaging experiments, NLX-112 showed dose-dependent reduction of 18F-F13640 (i.e., 18F-NLX-112) brain 5-HT1A receptor labeling in cingulate cortex and striatum, regions associated with motor control and mood, with almost complete inhibition of labeling at the dose of 0.63 mg/kg i.p.. Co-administration of L-DOPA (6 mg/kg s.c., a dose used to elicit LID in parkinsonian rats) together with NLX-112 (0.16 mg/kg i.p.) did not modify PK parameters in rat plasma and brain of either NLX-112 or L-DOPA. Here, we demonstrate that NLX-112's profile is compatible with 'druggable' parameters for CNS indications, and the results provide measures of brain concentrations and 5-HT1A receptor binding parameters relevant to the anti-dyskinetic activity of the compound.https://pubmed.ncbi.nlm.nih.gov/39096379/
|
| 参考文献 | |
| 其他信息 |
Serotonin 5-HT1 Receptor Agonists
Endogenous compounds and drugs that specifically stimulate SEROTONIN 5-HT1 RECEPTORS. Included under this heading are agonists for one or more of the specific 5-HT1 receptor subtypes.
Rationale: F13640 (befiradol) is a novel 5-HT(1A) receptor agonist with exceptional selectivity vs. other receptors and binding sites. It shows analgesic activity in animal models and is currently developed for human use. Objectives: Given the potential dual role of the serotonergic system in pain, through the modulation of ascending signals in spinal cord and their emotional processing by corticolimbic areas, we examined the in vivo activity of F13640 at somatodendritic autoreceptors and postsynaptic 5-HT(1A) heteroreceptors in medial prefrontal cortex (mPFC). Methods: In vivo single unit recordings and intracerebral microdialysis in the rat. Results: F13640 reduced the activity of dorsal raphe serotonergic neurons at 0.2-18.2 μg kg(-1), i.v. (cumulative doses; ED(50) = 0.69 μg kg(-1), i.v.) and increased the discharge rate of 80% of mPFC pyramidal neurons in the same dose range (ED(50) = 0.62 μg kg(-1), i.v.). Both effects were reversed by the subsequent administration of the 5-HT(1A) receptor antagonist (±)WAY100635. In microdialysis studies, F13640 (0.04-0.63 mg kg(-1), i.p.) dose-dependently decreased extracellular 5-HT in the hippocampus and mPFC. Likewise, F13640 (0.01-2.5 mg kg(-1), i.p.) dose-dependently increased extracellular DA in mPFC, an effect dependent on the activation of postsynaptic 5-HT(1A) receptors in mPFC. Local perfusion of F13640 in mPFC (1-1,000 μM) also increased extracellular DA in a concentration-dependent manner. Both the systemic and local effects of F13640 were prevented by prior (±)WAY100635 administration. Conclusions: These results indicate that, upon systemic administration, F13640 activates both 5-HT(1A) autoreceptors and postsynaptic 5-HT(1A) receptors in prefrontal cortex with a similar potency. Both activities are likely involved in the analgesic properties of the compound.[1] |
| 分子式 |
C20H22CLF2N3O
|
|---|---|
| 分子量 |
393.86
|
| 精确质量 |
393.142
|
| 元素分析 |
C, 60.99; H, 5.63; Cl, 9.00; F, 9.65; N, 10.67; O, 4.06
|
| CAS号 |
208110-64-9
|
| 相关CAS号 |
Befiradol hydrochloride;2436760-81-3
|
| PubChem CID |
9865384
|
| 外观&性状 |
Typically exists as white to off-white solids at room temperature
|
| 密度 |
1.3
|
| LogP |
4.245
|
| tPSA |
45.23
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
27
|
| 分子复杂度/Complexity |
502
|
| 定义原子立体中心数目 |
0
|
| SMILES |
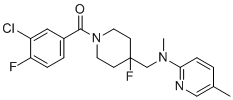
O=C(C1=CC=C(F)C(Cl)=C1)N2CCC(CNCC3=NC=C(C)C=C3)(F)CC2
|
| InChi Key |
PKZXLMVXBZICTF-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C20H22ClF2N3O/c1-14-2-4-16(25-11-14)12-24-13-20(23)6-8-26(9-7-20)19(27)15-3-5-18(22)17(21)10-15/h2-5,10-11,24H,6-9,12-13H2,1H3
|
| 化学名 |
(3-chloro-4-fluorophenyl)-[4-fluoro-4-[[(5-methylpyridin-2-yl)methylamino]methyl]piperidin-1-yl]methanone
|
| 别名 |
F-13640; F 13640; NLX-112; Befiradol (free base); Befiradol [INN]; RAT9OHA1YH; F13640; CHEMBL45305;F13640
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~102 mg/mL (~258.98 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5390 mL | 12.6949 mL | 25.3897 mL | |
| 5 mM | 0.5078 mL | 2.5390 mL | 5.0779 mL | |
| 10 mM | 0.2539 mL | 1.2695 mL | 2.5390 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03347331 | COMPLETED | Drug: [18F]F13640 Drug: [18F]F13640 |
Healthy Subjects Neurological Pathology |
Hospices Civils de Lyon | 2018-04-23 | Early Phase 1 |
| NCT05148884 | COMPLETEDWITH RESULTS | Drug: NLX-112 Drug: Placebo |
Medication-Induced Dyskinesia | Neurolixis SAS | 2021-11-09 | Phase 2 |
| NCT05084469 | UNKNOWN STATUS | Procedure: PET-MRI in pain-free remission period | Cluster Headache, Episodic | Hospices Civils de Lyon | 2021-11-01 |