| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g | |||
| Other Sizes |

| 靶点 |
Glucocorticoid Receptor (GR) [2][4]
- Nuclear Factor-kappa B (NF-κB) [2][4] |
|---|---|
| 体外研究 (In Vitro) |
在 L929 细胞中,倍他米松(0.1–1 μM;12 小时)可增加基因表达[4]。 CEM C7 T 细胞凋亡由倍他米松(0.1–1 μM;48 小时)诱导[4]。
在银屑病患者的人角质形成细胞和外周血单个核细胞(PBMCs)中,倍他米松(NSC-39470;SCH-4831)(1-100 nM)以剂量依赖方式抑制TNFα-IL-23-IL-17炎症轴。10 nM浓度时,RT-PCR检测显示TNFα mRNA表达降低55%、IL-23降低48%、IL-17降低62%,Western blot检测显示相应蛋白水平下调;50 nM浓度时抑制角质形成细胞过度增殖40%[3] - 在转染NF-κB依赖荧光素酶报告基因质粒的HeLa细胞中,倍他米松(NSC-39470;SCH-4831)(0.1-10 μM)抑制NF-κB介导的转录,1 μM浓度时最大抑制率达70%;10 μM浓度时诱导淋巴样细胞凋亡35%,5 μM浓度时糖皮质激素响应基因表达上调(如GRE驱动的荧光素酶活性增加2.3倍)[4] |
| 体内研究 (In Vivo) |
由于倍他米松(0.48 mg;IVGT 48 小时)可减少脑血管舒张,因此可减轻高碳酸血症引起的 CBF 增加[1]。局部给予倍他米松 (0.05 ml; 1 mg/L) 会降低大鼠脑中 NF-κB 的活化,升高 TNFα 和 IL-1β,并刺激 IL-10 的表达,所有这些都是由机械性异常性疼痛和热引起的脊髓神经横断引起的痛觉过敏[2]。
在妊娠晚期(128-132天)胎羊中,静脉注射倍他米松(NSC-39470;SCH-4831)(0.5 mg/kg,单次给药),给药后2小时内大脑皮层脑血流(CBF)增加30%、海马体脑血流增加25%,效应持续6小时,平均动脉压无显著变化[1] - 在慢性压迫性损伤(CCI)诱导的神经病变大鼠中,皮下注射倍他米松(NSC-39470;SCH-4831)(1 mg/kg,隔日一次,连续2周),机械性异常性疼痛减轻(足退缩阈值增加45%)、热痛觉过敏缓解(潜伏期延长38%);EMSA检测显示大脑皮层NF-κB活性降低50%,ELISA检测显示IL-1β(降低42%)和TNFα(降低39%)水平下降[2] - 在中重度银屑病患者中,外用钙泊三醇/倍他米松(NSC-39470;SCH-4831)软膏(每日1次,连续12周),银屑病面积和严重程度指数(PASI)较基线改善75%;皮肤活检显示表皮厚度减少60%,TNFα-IL-23-IL-17轴表达受抑(IL-17+ T细胞减少58%)[3] |
| 酶活实验 |
NF-κB转录活性检测:HeLa细胞共转染NF-κB响应荧光素酶报告基因质粒和海肾荧光素酶质粒(内参),24小时后加入0.1 μM、1 μM、10 μM的倍他米松(NSC-39470;SCH-4831),用TNFα刺激6小时。双荧光素酶检测系统测定荧光素酶活性,定量NF-κB转录抑制效果[4]
- 糖皮质激素响应基因激活检测:转染GRE驱动荧光素酶质粒的HeLa细胞,用0.5 μM、5 μM、10 μM的倍他米松(NSC-39470;SCH-4831)处理24小时,检测荧光素酶活性以评估GR介导的基因转录激活[4] |
| 细胞实验 |
银屑病相关炎症轴实验:银屑病患者的人角质形成细胞和PBMCs接种到6孔板,加入1 nM、10 nM、100 nM的倍他米松(NSC-39470;SCH-4831)培养48小时。提取总RNA,RT-PCR分析TNFα、IL-23、IL-17 mRNA水平;制备细胞裂解液,Western blot检测相应蛋白,以β-肌动蛋白为内参[3]
- 细胞凋亡实验:淋巴样细胞接种到96孔板,用1 μM、5 μM、10 μM的倍他米松(NSC-39470;SCH-4831)处理72小时。Annexin V-FITC/PI染色结合流式细胞术检测凋亡;MTT法检测细胞活力以排除非特异性细胞毒性[4] |
| 动物实验 |
Animal/Disease Models: Rambouillet-Colombia ewes bred on a single occasion are received hypercapnic challenges[1]
Doses: 0.48 mg Route of Administration: Injected into the fetal jugular vein at a rate of 1 ml/h (10 μg betamethasone/h) and maintained over the next 48 h. Experimental Results: diminished cerebral blood flow (CBF) in all brain regions measured except the hippocampus after 24 h of infusion. The reduction in CBF was diminished to about 25-30 % after 48 h of infusion. Fetal sheep cerebral blood flow model: Pregnant ewes (128-132 days of gestation) were anesthetized, and fetal sheep were instrumented with arterial catheters and cerebral blood flow probes. Betamethasone (NSC-39470; SCH-4831) (0.5 mg/kg) was administered intravenously to fetal sheep as a single dose. Cerebral blood flow in the cerebral cortex and hippocampus was measured at baseline, 1, 2, 4, and 6 hours post-dosing[1] - Rat CCI neuropathy model: Male Sprague-Dawley rats were subjected to CCI of the sciatic nerve to induce neuropathy. Starting 7 days post-surgery, Betamethasone (NSC-39470; SCH-4831) (1 mg/kg) was injected subcutaneously every other day for 2 weeks. Mechanical allodynia and thermal hyperalgesia were assessed weekly. Rats were euthanized, and cerebral cortex tissues were collected for NF-κB activity and cytokine detection[2] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The absorption and potency of any topical corticosteroid including betamethasone depends on the vehicle in which the steroid is delivered. For example, betamethasone dipropionate 0.05% ointment is classified as a highly potent topical steroid, while betamethasone dipropionate 0.05% cream or lotion is considered to be moderately potent. There are several structural modifications that can determine the potency of a topical corticosteroid. For example, corticosteroids containing a halogen at specific carbons, or that contain esters are more potent due to enhanced lipophilicity. As such, there is a marked difference between topical products containing betamethasone dipropionate vs. betamethasone valerate. Betamethasone dipropionate contains 2 esters which enhances its potency, while betamethasone valerate has only one ester and is less potent. It should be noted that the use of occlusive dressings with topical steroids significantly increases the absorption, increasing the risk for adverse effects. Corticosteroids are eliminated predominantly in the urine. In a study that included Indian women of reproductive age, the volume of distribution following a single intramuscular dose of betamethasone phosphate was 94,584±23,539 mL(s). In a study that included Indian women of reproductive age, the CL/F following a single intramuscular dose of betamethasone phosphate was 6,466 ± 805 mL/hour. Glucocorticoids ... absorbed systemically from sites of local administration, such as synovial spaces, the conjunctival sac, skin, and respiratory tract. When administration is prolonged, when the site of application is covered with an occlusive dressing, or when large areas of skin are involved, the absorption may be sufficient to cause systemic effects, including suppression of the HPA axis. /Adrenocorticalsteroids/ Following absorption, 90% or more of cortisol in plasma is reversibly bound to protein under normal circumstances. Only the fraction of corticosteroid that is unbound can enter cells to mediate corticosteroid effects. Two plasma proteins account for almost all of the steroid-binding capacity: corticosteroid-binding globulin (CBG; also called transcortin), and albumin. CBG is an alpha-globulin secreted by the liver that has high affinity for steroids but relatively low total binding capacity, whereas albumin, also produced by the liver, has low affinity but relatively large binding capacity. At normal or low concentrations of corticosteroids, most of the hormone is protein-bound. At higher steroid concentrations, the capacity of protein binding is exceeded, and a significantly greater fraction of the steroid exists in the free state. Corticosteroids compete with each other for binding sites on CBG. CBG has relatively high affinity for cortisol and most of its synthetic congeners and low affinity for aldosterone and glucuronide-conjugated steroid metabolites; thus, greater percentages of these latter steroids are found in the free form. /Adrenocortical Steroids/ The pharmacokinetics of betamethasone and its phosphate ester are described in 8 healthy adults after iv bolus injection of 10.6 mg betamethasone phosphate. Both cmpd were measured by high performance liquid chromatography with ultraviolet detection using sample handling methods which prevented hydrolysis of the ester in vitro. Betamethasone phosphate disappeared rapidly from plasma (mean half-life = 4.7 min) as betamethasone levels rose. Betamethasone plasma levels reached a peak 10-36 min after admin of the phosphate before declining in a biexponential manner. The terminal slow disposition phase had a mean half-life of 6.5 hr. Only about 5% of the dose was recovered from urine as betamethasone, indicating extensive extrarenal clearance of betamethasone. /Betamethason phosphate/ Metabolism / Metabolites The metabolism of betamethasone yields 6 metabolites. The metabolic processes include 6β hydroxylation, 11β-hydroxyl oxidation, and reduction of the C-20 carbonyl group followed by removal of the side chain. All of the biologically active adrenocortical steroids and their synthetic congeners have a double bond in the 4,5 position and a ketone group at C 3. As a general rule, the metabolism of steroid hormones involves sequential additions of oxygen or hydrogen atoms, followed by conjugation to form water-soluble derivatives. Reduction of the 4,5 double bond occurs at both hepatic and extrahepatic sites, yielding inactive compounds. Subsequent reduction of the 3-ketone substituent to the 3-hydroxyl derivative, forming tetrahydrocortisol, occurs only in the liver. Most of these A ring-reduced steroids are conjugated through the 3-hydroxyl group with sulfate or glucuronide by enzymatic reactions that take place in the liver and, to a lesser extent, in the kidney. The resultant sulfate esters and glucuronides form water-soluble derivatives and are the predominant forms excreted in the urine. Neither biliary nor fecal excretion is of quantitative importance in human beings. /Adrenocortical Steroids/ Biological Half-Life In a study that included Indian women of reproductive age, the half-life following a single intramuscular dose of betamethasone phosphate was 10.2 ± 2.5 hours. The pharmacokinetics of betamethasone and its phosphate ester are described in 8 healthy adults after i.v. bolus injection of 10.6 mg betamethasone phosphate. Both compounds were measured by high-performance liquid chromatography with ultraviolet detection using sample handling methods which prevented hydrolysis of the ester in vitro. Betamethasone phosphate disappeared rapidly from plasma (mean half-life = 4.7 min) as betamethasone levels rose. Betamethasone plasma levels reached a peak 10-36 min after administration of the phosphate before declining in a biexponential manner. The terminal slow disposition phase had a mean half-life of 6.5 hr. Serum half-life of betamethasone is about 3 hr. |
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
Hyperglycemic action of cortisone /betamethasone/ may offset hypoglycemic effect of chlorpropamide... Induction of hepatic enzymes by corticosteroids may increase the formation of a hepatotoxic acetaminophen metabolite, thereby increasing the risk of hepatotoxicity, when they are used concurrently with chronic or high-dose acetaminophen therapy. /Corticosteroid/ Risk of gastrointestinal ulceration or hemorrhage may be increased when these substances /alcohol or nonsteroidal anti-inflammatory drugs (NSAIDs)/ are used concurrently with glucocorticoids; however, concurrent use of NSAIDs in the treatment of arthritis may provide additive therapeutic benefit and permit glucocorticoids dosage reduction. /Corticosteroids/ Concurrent use /of carbonic anhydrase inhibitors/ with corticosteroids may result in severe hypokalemia and should be undertaken with caution; serum potassium concentrations and cardiac function should be monitored during concurrent use. /Corticosteroids/ For more Interactions (Complete) data for BETAMETHASONE (25 total), please visit the HSDB record page. Local toxicity: Topical application in psoriasis patients showed mild skin irritation (incidence 8%), including erythema and pruritus, which resolved spontaneously without treatment interruption[3] - Systemic toxicity: In fetal sheep and rat models, no significant changes in vital signs (mean arterial pressure, heart rate) or organ function markers (ALT, AST, creatinine) were observed at therapeutic doses[1][2] - Plasma protein binding rate: ~98% bound to human plasma proteins[3] |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Anti-Asthmatic Agents; Anti-Inflammatory Agents, Steroidal; Glucocorticoids, Synthetic; Glucocorticoids, Topical /Indicated for the treament of/ allergic disorders: drug induced allergic reactions; angioedema; acute noninfectious laryngeal edema; allergic, perennial or seasonal, severe rhinitis; serum sickness; urticarial transfusions reactions. /Indicated for the treament of/ collagen disorders: acute, rheumatic or nonrheumatic carditis; systemic lupus erythematosus; mixed connective tissue disease; polyarteritis nodosa; relapsing polychondritis. /Indicated for the treatment of/ dermatologic disorders: alopecia areata; atopic dermatitis; contact dermatitis; exfoliative dermatitis; herpetiformis, bullous dermatitis; severe, seborrheic dermatitis; severe inflammatory dermatoses; severe multiforme erythema; granuloma annulare; keloids; lichen planus; lichen simplex chronicus; discoid lupus erythematosus; mycosis fungoides; necrobiosis lipoidica diabeticorum; pemphigus; severe psoriasis; psoriatic plaques; severa eczema; pemphigoid; localized cutaneous sarcoid. For more Therapeutic Uses (Complete) data for BETAMETHASONE (16 total), please visit the HSDB record page. Drug Warnings ... The most striking effects of corticosteroids on the cardiovascular system result from mineralocorticoid-induced changes in renal Na + excretion as is evident in primary aldosteronism. The resultant hypertension can lead to a diverse group of adverse effects on the cardiovascular system, including increased atherosclerosis, cerebral hemorrhage, stroke, and hypertensive cardiomyopathy. The mechanism underlying the hypertension remains incompletely understood, but restriction of dietary Na + can lower the blood pressure considerably. /Adrenocorticosteroids/ Two effects of corticosteroids on lipid metabolism are firmly established. The first is the dramatic redistribution of body fat that occurs in settings of hypercorticism such as Cushing's syndrome. The other is the permissive facilitation of the effect of other agents, such as growth hormone and beta-adrenergic receptor agonists, in inducing lipolysis in adipocytes, with a resultant increase in free fatty acids following glucocorticoid administration. /Adrenocorticosteroids/ ...Caution should be exercised if fluorinated preparations are used on face or other cosmetically important areas, since paradoxical eruptions may occur with long-term use. Although injection may be given intra-articularly, it must be remembered that repeated intra-articular glucocorticoids sometimes effect painless destruction of joint. For more Drug Warnings (Complete) data for BETAMETHASONE (34 total), please visit the HSDB record page. Pharmacodynamics Corticosteroids bind to the glucocorticoid receptor inhibiting pro-inflammatory signals, while promoting anti-inflammatory signals. Corticosteroids have a wide therapeutic window as patients may require doses that are multiples of what the body naturally produces. Patients who require long-term treatment with a corticosteroid should be counselled regarding the risk of hypothalamic-pituitary-adrenal axis suppression and increased susceptibility to infections. Betamethasone (NSC-39470; SCH-4831) is a synthetic glucocorticoid with potent anti-inflammatory, immunosuppressive, and anti-proliferative properties[2][3][4] - Its core mechanisms include binding to GR to mediate transactivation of anti-inflammatory genes and transrepression of pro-inflammatory genes (e.g., TNFα, IL-23, IL-17), repressing NF-κB-dependent transcription, and inducing apoptosis of pro-inflammatory cells[3][4] - Clinical indications include psoriasis (topical combination therapy), neurogenic pain, and fetal lung maturation promotion (off-label use in preterm labor)[1][2][3] - It exhibits tissue-specific effects, such as increasing fetal cerebral blood flow without systemic hemodynamic disturbance, and suppressing the TNFα-IL-23-IL-17 axis critical for psoriasis pathogenesis[1][3] - Compared to other glucocorticoids, it has high potency in repressing NF-κB activity and inducing glucocorticoid-responsive genes, contributing to its therapeutic efficacy in inflammatory and neuropathic conditions[4] |
| 分子式 |
C22H29FO5
|
|
|---|---|---|
| 分子量 |
392.46
|
|
| 精确质量 |
392.199
|
|
| CAS号 |
378-44-9
|
|
| 相关CAS号 |
Betamethasone disodium phosphate;151-73-5;Betamethasone-d5;Betamethasone dipropionate;5593-20-4;Betamethasone valerate;2152-44-5;Betamethasone hydrochloride;956901-32-9;Betamethasone acetate;987-24-6;Betamethasone-d5-1;2244574-92-1
|
|
| PubChem CID |
9782
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
568.2±50.0 °C at 760 mmHg
|
|
| 熔点 |
235-237°C
|
|
| 闪点 |
297.5±30.1 °C
|
|
| 蒸汽压 |
0.0±3.5 mmHg at 25°C
|
|
| 折射率 |
1.592
|
|
| LogP |
1.87
|
|
| tPSA |
94.83
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
2
|
|
| 重原子数目 |
28
|
|
| 分子复杂度/Complexity |
805
|
|
| 定义原子立体中心数目 |
8
|
|
| SMILES |
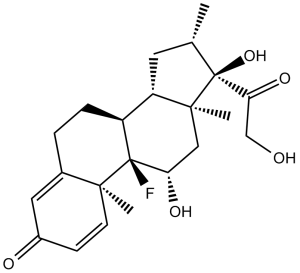
C[C@H]1C[C@H]2[C@@H]3CCC4=CC(=O)C=C[C@@]4([C@]3([C@H](C[C@@]2([C@]1(C(=O)CO)O)C)O)F)C
|
|
| InChi Key |
UREBDLICKHMUKA-DVTGEIKXSA-N
|
|
| InChi Code |
InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15-,16-,17-,19-,20-,21-,22-/m0/s1
|
|
| 化学名 |
(8S,9R,10S,11S,13S,14S,16S,17R)-9-fluoro-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.37 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.37 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.37 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5480 mL | 12.7402 mL | 25.4803 mL | |
| 5 mM | 0.5096 mL | 2.5480 mL | 5.0961 mL | |
| 10 mM | 0.2548 mL | 1.2740 mL | 2.5480 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Does a Rescue Course of Betamethasone in Pregnant Women With PPROM Decrease Neonatal Morbidity?
CTID: NCT02939742
Phase: Phase 2/Phase 3 Status: Terminated
Date: 2024-01-26