| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 体外研究 (In Vitro) |
Amfebutamone,也称为安非他酮,抑制 CYP2D6,IC50 为 58 μM[1]。安非他酮等非典型抗抑郁药会在 SH-SY5Y 细胞中引起内质网应激和依赖于 caspase 的细胞毒性 [3]。通过触发内质网应激反应和 JNK 激活,安非他酮激活 caspase 3,导致 SH-SY5Y 细胞凋亡 [3]。 1–100 µg/mL 的安非他酮会降低细胞活力。细胞凋亡途径可能是安非他酮诱导的细胞活力降低的原因[3]。一小时内,安非他酮 (100 μg/mL) 增强 JNK、p38 MAPK、EIF-2α 和 GRP78 磷酸化版本的表达 [3]。
|
|---|---|
| 体内研究 (In Vivo) |
在小鼠中,安非他酮表现出惊厥和抗惊厥特性。安非他酮引起小鼠剂量依赖性阵挛性惊厥,惊厥剂量 50(或 CD50)为 119.7 mg/kg,在该水平下 50% 的小鼠出现惊厥[4]。在体重为 22-30 g 的雄性白化小鼠中,与载体对照(以秒为单位)相比,安非他酮(10、15、20 和 40 mg/kg,腹膜内注射)剂量依赖性地减少了不动时间。在强迫游泳试验和悬尾试验中观察到安非他酮可减少不动期,ED50 值分别为 18.5 和 18 mg/kg ip。安非他酮(10、20 和 40 mg/kg,腹腔注射)以剂量依赖性方式提高小鼠大脑中高香草酸(游离多巴胺的代谢产物)的浓度 [5]。
|
| 细胞实验 |
细胞活力测定 [3]
细胞类型: SH-SY5Y 人儿茶酚胺能细胞 测试浓度: 0、1、10、50 和 100 µg/mL 孵育持续时间:24小时 实验结果:细胞活力以浓度依赖性方式急剧下降。 蛋白质印迹分析 [3] 细胞类型: SH-SY5Y 人儿茶酚胺能细胞 测试浓度: 100 µg/mL 孵育持续时间:1、3、8、24 小时 实验结果:安非他酮 1 小时内对 p-EIF-2α 产生免疫力治疗反应性显着增加并持续3小时,表明安非他酮快速刺激PERK。 GRP78的表达轻微但显着增加并且JNK显着激活。安非他酮对 ER 应激通路的早期激活在治疗后 8 小时恢复到基础水平。 |
| 动物实验 |
Animal/Disease Models: Male Swiss mouse, weight 20-25 grams [4]
Doses: 100-160 mg/kg Route of Administration: IP Experimental Results:Caused clonic convulsions, CD50 and CD97 were 119.7 (104.1-137.6) and 156.7 respectively mg/kg. When administered at the full convulsive dose of 160 mg/kg, the median latency was 6.00 minutes (3.50-8.15). Catalonic convulsions were only observed occasionally (1 in 8 mice) in groups receiving doses of 140 or 160 mg/kg. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Bupropion is currently available in 3 distinct, but bioequivalent formulations: immediate release (IR), sustained-release (SR), and extended-release (XL). **Immediate Release Formulation** In humans, following oral administration of bupropion hydrochloride tablets, peak plasma bupropion concentrations are usually achieved within 2 hours. IR formulations provide a short duration of action and are therefore generally dosed three times per day. **Sustained Release Formulation** In humans, following oral administration of bupropion hydrochloride sustained-release tablets (SR), peak plasma concentration (Cmax) of bupropion is usually achieved within 3 hours. SR formulations provide a 12-hour extended release of medication and are therefore generally dosed twice per day. **Extended Release Formulation** Following single oral administration of bupropion hydrochloride extended-release tablets (XL) to healthy volunteers, the median time to peak plasma concentrations for bupropion was approximately 5 hours. The presence of food did not affect the peak concentration or area under the curve of bupropion. XL formulations provide a 24-hour extended release of medication and are therefore generally dosed once per day/ In a trial comparing chronic dosing with bupropion hydrochloride extended-release tablets (SR) 150 mg twice daily to bupropion immediate-release formulation 100 mg 3 times daily, the steady state Cmax for bupropion after bupropion hydrochloride sustained-release tablets (SR) administration was approximately 85% of those achieved after bupropion immediate-release formulation administration. Exposure (AUC) to bupropion was equivalent for both formulations. Bioequivalence was also demonstrated for all three major active metabolites (i.e., hydroxybupropion, threohydrobupropion and erythrohydrobupropion) for both Cmax and AUC. Thus, at steady state, bupropion hydrochloride sustained-release tablets (SR) given twice daily, and the immediate-release formulation of bupropion given 3 times daily, are essentially bioequivalent for both bupropion and the 3 quantitatively important metabolites. Furthermore, in a study comparing 14-day dosing with bupropion hydrochloride extended-release tablets (XL), 300 mg once-daily to the immediate-release formulation of bupropion at 100 mg 3 times daily, equivalence was demonstrated for peak plasma concentration and area under the curve for bupropion and the three metabolites (hydroxybupropion, threohydrobupropion, and erythrohydrobupropion). Additionally, in a study comparing 14-day dosing with bupropion hydrochloride extended-release tablets (XL) 300 mg once daily to the sustained-release formulation of bupropion at 150 mg 2 times daily, equivalence was demonstrated for peak plasma concentration and area under the curve for bupropion and the three metabolites. Bupropion hydrochloride extended-release tablets (SR) can be taken with or without food. Bupropion Cmax and AUC were increased by 11% to 35% and 16% to 19%, respectively, when bupropion hydrochloride extended-release tablets (SR) was administered with food to healthy volunteers in three trials. The food effect is not considered clinically significant. Following a single-dose administration of bupropion hydrochloride extended-release tablets (SR) in humans, Cmax of bupropion's metabolite hydroxybupropion occurs approximately 6 hours post-dose and is approximately 10 times the peak level of the parent drug at steady state. The elimination half-life of hydroxybupropion is approximately 20 (±5) hours and its AUC at steady state is about 17 times that of bupropion. The times to peak concentrations for the erythrohydrobupropion and threohydrobupropion metabolites are similar to that of the hydroxybupropion metabolite. However, their elimination half-lives are longer, 33(±10) and 37 (±13) hours, respectively, and steady-state AUCs are 1.5 and 7 times that of bupropion, respectively. Bupropion is extensively metabolized in humans. Oxidation of the bupropion side chain results in the formation of a glycine conjugate of metachlorobenzoic acid, which is then excreted as the major urinary metabolite. Following oral administration of 200 mg of 14C-bupropion in humans, 87% and 10% of the radioactive dose were recovered in the urine and feces, respectively. However, the fraction of the oral dose of bupropion excreted unchanged was only 0.5%, a finding consistent with the extensive metabolism of bupropion. Peak plasma bupropion concentrations usually occur within 2 or 3 hours after oral administration of the conventional or extended-release, film-coated tablets (Wellbutrin SR, Zyban), respectively, to healthy individuals. Plasma bupropion concentrations following administration of single oral doses of 100-250 mg and with chronic administration of up to 450 mg daily are proportional to dose. Steady-state plasma concentrations of bupropion are achieved within 8 days. During chronic administration of bupropion hydrochloride as conventional or extended-release, film-coated tablets at a dosage of 100 mg 3 times daily or 150 mg twice daily, respectively, peak plasma concentrations of the drug at steady state with extended-release tablets were about 85% of measurements for the conventional tablets. Equivalence in area under the plasma concentration-time curve (AUC) of bupropion was shown for the formulations, which demonstrated that at steady state the conventional and extended-release tablets are essentially bioequivalent. The drug exhibits linear pharmacokinetics during chronic administration of bupropion hydrochloride dosages of 300-450 mg daily. Bupropion hydrochloride appears to be well absorbed from the GI tract following oral administration. The oral bioavailability of bupropion in humans has not been elucidated because a preparation for IV administration is not available. However, the relative proportion of an oral dose reaching systemic circulation unchanged appears likely to be small. In animals, the oral bioavailability of bupropion varies from 5-20%. Food does not appear to affect substantially the peak plasma concentration or area under the plasma concentration-time curve of bupropion achieved with extended-release tablets of the drug; these measures reportedly were increased with food by 11 or 17%, respectively. Approximately 87 and 10% of an orally administered, radiolabeled dose of bupropion are excreted in urine and feces, respectively. Unchanged drug comprised 0.5% of the dose excreted. Plasma concentrations of bupropion decline in a biphasic manner.1 57 61 130 A decline to approximately 30% of the peak plasma bupropion concentration is observed 6 hours after administration of a single oral dose of the drug. For more Absorption, Distribution and Excretion (Complete) data for Bupropion (9 total), please visit the HSDB record page. Metabolism / Metabolites Bupropion is extensively metabolized in humans. Three metabolites are active: hydroxybupropion, which is formed via hydroxylation of the tert-butyl group of bupropion, and the amino-alcohol isomers, threohydrobupropion and erythrohydrobupropion, which are formed via reduction of the carbonyl group. In vitro findings suggest that CYP2B6 is the principal isoenzyme involved in the formation of hydroxybupropion, while cytochrome P450 enzymes are not involved in the formation of threohydrobupropion. Hydroxybupropion has been shown to have the same affinity as bupropion for the norepinephrine transporter (NET) but approximately 50% of its antidepressant activity despite reaching concentrations of ~10-fold higher than that of the parent drug. Oxidation of the bupropion side chain results in the formation of a glycine conjugate of meta-chlorobenzoic acid, which is then excreted as the major urinary metabolite. The potency and toxicity of the metabolites relative to bupropion have not been fully characterized. However, it has been demonstrated in an antidepressant screening test in mice that hydroxybupropion is one-half as potent as bupropion, while threohydrobupropion and erythrohydrobupropion are 5-fold less potent than bupropion. This may be of clinical importance because the plasma concentrations of the metabolites are as high as or higher than those of bupropion. Bupropion and its metabolites exhibit linear kinetics following chronic administration of 300 to 450 mg per day. Bupropion appears to be metabolized extensively, probably in the liver. The 3 active metabolites that have been identified are formed through reduction of the carbonyl group and/or hydroxylation. The basic metabolites identified include the erythro- and threo-amino alcohols of bupropion, and a morpholinol metabolite. The amino-alcohol isomers threohydrobupropion and erythrohydrobupropion are formed by reduction of the carbonyl group of bupropion, and the morpholinol metabolite, hydroxybupropion, is formed by hydroxylation of the tert-butyl group of bupropion. The metabolites of bupropion exhibit linear pharmacokinetics during chronic administration of the drug at dosages of 300-450 mg daily. All available antidepressants with the exception of fluvoxamine and nefazodone either are metabolized by cytochrome P450 2D6 (CYP2D6) and/or inhibit this isozyme. To date, nothing in this regard has been published concerning bupropion. We report that plasma level/dose ratios for bupropion, and its metabolites erythrohydrobupropion and threohydrobupropion, were not associated with debrisoquine metabolic status in 12 patients, three of whom were poor 2D6 metabolizers. The plasma level/dose ratios for the metabolite hydroxybupropion were, however, significantly higher in poor 2D6 metabolizers. In three patients, who received a second phenotyping test during treatment with bupropion, debrisoquine metabolic ratios were not increased. It is thus inferred that bupropion is neither metabolized by nor inhibits CYP2D6. The potential accumulation of hydroxybupropion after CYP2D6 inhibition may, however, contribute to toxicity and impair bupropion's therapeutic effectiveness Bupropion hydrochloride is a new monocyclic antidepressant. In humans, its disposition results in the formation of three major metabolites: the morpholinol metabolite, the erythroamino alcohol, and the threoamino alcohol metabolite. Bupropion's disposition was monitored following a single oral 200 mg dose in eight healthy volunteers and eight age- (44.5 +/- 8.4 years) and weight- (77.4 +/- 6.7 kg) matched volunteers with alcoholic liver disease. This latter group is of interest because the incidence of depression is more frequent in alcoholics than in the general population, and the liver is the major route of elimination for cyclic antidepressants. The mean elimination half-life of the morpholinol metabolite was significantly prolonged in subjects with alcoholic liver disease (32.2 +/- 13.5 vs. 21.1 +/- 4.9 hours (p less than 0.05), while the differences in bupropion (17.3 +/- 8.6 hours vs. 16.5 +/- 10.4 hours for healthy subjects and subjects with alcoholic liver disease, respectively), erythroamino alcohol (26.1 +/- 13.3 hours vs. 29.8 +/- 6.9 hours for healthy subjects and subjects with alcoholic liver disease, respectively), and threoamino alcohol (25.5 +/- 8.6 hours vs. 23.4 +/- 10.7 hours for healthy subjects and subjects with alcoholic liver disease, respectively) were minimal. Mean area under the plasma concentration time curves for bupropion and metabolites were increased in subjects with alcoholic liver disease; however, clear differences between means of these small groups did not emerge, probably due to the increased variability of bupropion pharmacokinetics in these subjects. As a therapeutic agent for the treatment of depression in chronic alcoholics who may consume alcohol in combination with their antidepressant therapy, the lack of sedation with bupropion could be advantageous. We studied the steady-state pharmacokinetics of bupropion hydrochloride, a unicyclic aminoketone antidepressant, in depressed patients. The metabolites hydroxybupropion (HB), threohydrobupropion, and erythrohydrobupropion predominated over the parent compound in plasma and cerebrospinal fluid at steady state. Plasma concentrations of each metabolite correlated with cerebrospinal fluid concentrations. Higher plasma metabolite concentrations were associated with poor clinical outcome. This relationship was most striking with HB; plasma HB levels were greater than 1250 ng/mL in all five nonresponders and less than 1200 ng/mL in all seven responders. Plasma HB levels correlated with postreatment plasma homovanillic acid levels. High levels of bupropion metabolites may be associated with poor clinical outcome due to toxic effects involving dopaminergic systems. Alternatively, a curvilinear dose-response relationship may exist for bupropion metabolites. Future studies should explore the clinical utility of plasma metabolite measurements in enhancing the efficacy of treatment with bupropion. For more Metabolism/Metabolites (Complete) data for Bupropion (9 total), please visit the HSDB record page. Reduction of the carbonyl groupand/or hydroxylation of the tert-butyl group of bupropion. Route of Elimination: Bupropion is extensively metabolized in humans. Oxidation of the bupropion side chain results in the formation of a glycine conjugate of metachlorobenzoic acid, which is then excreted as the major urinary metabolite. Following oral administration of 200 mg of 14C-bupropion in humans, 87% and 10% of the radioactive dose were recovered in the urine and feces, respectively. However, the fraction of the oral dose of bupropion excreted unchanged was only 0.5%, a finding consistent with the extensive metabolism of bupropion. Half Life: 24 hours Biological Half-Life 24 hours The half-life of bupropion in the terminal phase averages about 14 hours (range: 8-24 hours) following single doses; with multiple dosing, the half-life of bupropion in the terminal phase reportedly averages 21 hours (range: 8-39 hours). In a limited number of geriatric patients with a major depressive episode, the half-life of bupropion in the terminal phase averaged about 34 hours after a single oral dose of the drug. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Bupropion is pale yellow oil formulated in extended release oral tablets. Bupropion is a dopamine uptake inhibitor, which is used as a second generation antidepressive agent. HUMAN EXPOSURE AND TOXICITY: Bupropion is an antidepressant commonly prescribed as a smoking cessation aid. It has effects on dopamine and norepinephrine, and can lower seizure threshold, particularly in overdose. Several cases of recreational use of bupropion via nasal insufflation have been reported in the literature. One of the potentially most serious adverse effects of bupropion is reduction in the seizure threshold. However, despite the potential seriousness of this effect, seizures remain a relatively uncommon adverse effect of bupropion therapy. Patients reportedly have overdosed with 30 g or more of bupropion hydrochloride. Serious effects of overdosage have included seizures in about one-third of such patients, hallucinations, loss of consciousness, sinus tachycardia, and ECG changes such as conduction disturbances or arrhythmias. Lethargy, grogginess, tremors, jitteriness, confusion, lightheadedness, paresthesias, visual hallucinations, blurred vision, nausea, and vomiting also have occurred. Overdosage of bupropion (mainly as part of multiple drug overdoses) reportedly has resulted in fever, muscle rigidity, rhabdomyolysis, hypotension, stupor, coma, and respiratory failure. Recovery without sequelae has been reported in most individuals following an overdose of bupropion alone. However, massive overdosage of bupropion alone has been reported rarely to result in death preceded by multiple uncontrolled seizures, bradycardia, cardiac failure, and cardiac arrest. Cardiotoxicity appears to be caused primarily by bupropion rather than its active metabolite hydroxybupropion. Unintentional ingestion of bupropion in young children has generally resulted in limited toxicity. In two deaths attributed to bupropion, the doses in both cases were estimated to be less than 10 g. ANIMAL STUDIES: In lifetime carcinogenicity studies of rats or mice receiving bupropion hydrochloride dosages of 100-300 or 150 mg/kg daily respectively, an increase in nodular proliferative lesions of the liver was observed in rats but not in mice. The relationship of these lesions to the development of neoplasms of the liver is unclear. An increase in malignant tumors of the liver and other organs was not observed in either rats or mice. A fertility study in rats using oral bupropion hydrochloride dosages of up to 300 mg/kg daily did not reveal evidence of impaired fertility. In rats receiving oral dosages of bupropion of up to 300 mg/kg daily prior to mating and throughout pregnancy and lactation, there were no apparent adverse effects on offspring development. In developmental studies performed in rats and rabbits, no clear evidence of teratogenic activity was found in either species, but slightly increased incidences of fetal malformations and skeletal variations were observed in rabbits. Bupropion induced behavioral changes in rats and mice. Bupropion exhibited mutagenic activity in the Salmonella microbial mutagen (Ames) test system; the mutation rate was 2-3 times control in 2 of 5 strains. An increase in chromosomal aberrations was observed in one of 3 in vivo cytogenetic studies conducted with the bone marrow of rats. Bupropion selectively inhibits the neuronal reuptake of dopamine, norepinephrine, and serotonin; actions on dopaminergic systems are more significant than imipramine or amitriptyline whereas the blockade of norepinephrine and serotonin reuptake at the neuronal membrane is weaker for bupropion than for tricyclic antidepressants. The increase in norepinephrine may attenuate nicotine withdrawal symptoms and the increase in dopamine at neuronal sites may reduce nicotine cravings and the urge to smoke. Bupropion exhibits moderate anticholinergic effects. Interactions Adverse neuropsychiatric events or reduced alcohol tolerance have been reported rarely in patients who ingested alcohol during bupropion therapy. Because of concerns that excessive use of alcohol or abrupt withdrawal from alcohol may be associated with an increased risk of seizures during bupropion therapy, patients receiving the drug should be advised to minimize or, if possible, avoid alcohol consumption. Concomitant administration of bupropion and carbamazepine resulted in decreases in the peak plasma concentration of bupropion and in the 24-hour area under the plasma concentration-time curve (AUC) by 87 and 90%, respectively; the peak plasma concentration and 24-hour AUC of the metabolite, hydroxybupropion, were increased by 71 and 50%, respectively.138 In contrast, concomitant administration of bupropion and valproate sodium resulted only in an increase by 94% in 24-hour AUC of hydroxybupropion.138 A limited number of patients with parkinsonian syndrome treated with either amantadine or levodopa appeared to have a high incidence of adverse effects (e.g., nausea and vomiting, excitement and restlessness, postural tremor) when bupropion was used concurrently. Caution should be exercised if bupropion therapy is initiated in a patient receiving levodopa or amantadine, including use of low initial dosage and increasing the dosage gradually in small increments. Evidence from animal studies suggests that concomitant administration of bupropion and monoamine oxidase (MAO) inhibitors is potentially hazardous. In animals, phenelzine enhanced the acute toxicity of bupropion, as indicated by an increase in mortality and a decrease in time to death. The manufacturer states that concurrent administration of bupropion and MAO inhibitors is contraindicated and that at least 2 weeks elapse following discontinuance of an MAO inhibitor prior to initiation of bupropion therapy For more Interactions (Complete) data for Bupropion (20 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Mouse ip 230 mg/kg LD50 Mouse oral 575 mg/kg LD50 Rat ip 210 mg/kg LD50 Rat oral 600 mg/kg |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Antidepressive Agents, Second-Generation; Dopamine Uptake Inhibitors Zyban is indicated as an aid to smoking cessation treatment. /Included in US product label/ Wellbutrin XL is indicated for the treatment of major depressive disorder. /Included in US product labeling/ Wellbutrin XL is indicated for the prevention of seasonal major depressive episodes in patients with a diagnosis of seasonal affective disorder. For more Therapeutic Uses (Complete) data for Bupropion (9 total), please visit the HSDB record page. Drug Warnings /BOXED WARNING/ WARNING: NEUROPSYCHIATRIC REACTIONS IN PATIENTS TAKING BUPROPION FOR SMOKING CESSATION. Serious neuropsychiatric reactions have occurred in patients taking Zyban for smoking cessation. The majority of these reactions occurred during bupropion treatment, but some occurred in the context of discontinuing treatment. In many cases, a causal relationship to bupropion treatment is not certain, because depressed mood may be a symptom of nicotine withdrawal. However, some of these symptoms have occurred in patients taking Zyban who continued to smoke. The risks of Zyban should be weighed against the benefits of its use. Zyban has been demonstrated to increase the likelihood of abstinence from smoking for as long as 6 months compared with treatment with placebo. The health benefits of quitting smoking are immediate and substantial. /BOXED WARNING/ WARNING: SUICIDALITY AND ANTIDEPRESSANT DRUGS. Although Zyban is not indicated for treatment of depression, it contains the same active ingredient as the antidepressant medications Wellbutrin, Wellbutrin SR, and Wellbutrin XL. Antidepressants increased the risk of suicidal thoughts and behavior in children, adolescents, and young adults in short-term trials. These trials did not show an increase in the risk of suicidal thoughts and behavior with antidepressant use in subjects over age 24; there was a reduction in risk with antidepressant use in subjects aged 65 and older. In patients of all ages who are started on antidepressant therapy, monitor closely for worsening, and for emergence of suicidal thoughts and behaviors. Advise families and caregivers of the need for close observation and communication with the prescriber. /BOXED WARNING/ WARNING: SUICIDALITY AND ANTIDEPRESSANT DRUGS. Use in Treating Psychiatric Disorders: Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of Wellbutrin XL or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Wellbutrin XL is not approved for use in pediatric patients. /BOXED WARNING/ WARNING: Use in Smoking Cessation Treatment: Wellbutrin, Wellbutrin SR and Wellbutrin XL are not approved for smoking cessation treatment, but bupropion under the name Zyban is approved for this use. Serious neuropsychiatric events, including but not limited to depression, suicidal ideation, suicide attempt, and completed suicide have been reported in patients taking bupropion for smoking cessation. Some cases may have been complicated by the symptoms of nicotine withdrawal in patients who stopped smoking. Depressed mood may be a symptom of nicotine withdrawal. Depression, rarely including suicidal ideation, has been reported in smokers undergoing a smoking cessation attempt without medication. However, some of these symptoms have occurred in patients taking bupropion who continued to smoke. All patients treated with bupropion for smoking cessation treatment should be observed for neuropsychiatric symptoms including changes in behavior, hostility, agitation, depressed mood, and suicide-related events, including ideation, behavior, and attempted suicide. These symptoms, as well as worsening of pre-existing psychiatric illness and completed suicide have been reported in some patients attempting to quit smoking while taking Zyban in the post-marketing experience. When symptoms were reported, most were during treatment with Zyban, but some were following discontinuation of treatment with Zyban. These events have occurred in patients with and without pre-existing psychiatric disease; some have experienced worsening of their psychiatric illnesses. Patients with serious psychiatric illness such as schizophrenia, bipolar disorder, and major depressive disorder did not participate in the pre-marketing studies of Zyban. Advise patients and caregivers that the patient using bupropion for smoking cessation should stop taking bupropion and contact a healthcare provider immediately if agitation, hostility, depressed mood, or changes in thinking or behavior that are not typical for the patient are observed, or if the patient develops suicidal ideation or suicidal behavior. In many post-marketing cases, resolution of symptoms after discontinuation of Zyban was reported, although in some cases the symptoms persisted; therefore, ongoing monitoring and supportive care should be provided until symptoms resolve. The risks of using bupropion for smoking cessation should be weighed against the benefits of its use. Zyban has been demonstrated to increase the likelihood of abstinence from smoking for as long as six months compared to treatment with placebo. The health benefits of quitting smoking are immediate and substantial. For more Drug Warnings (Complete) data for Bupropion (41 total), please visit the HSDB record page. Pharmacodynamics Bupropion is chemically unrelated to tricyclic, tetracyclic, selective serotonin re-uptake inhibitors, or other known antidepressant agents. Compared to classical tricyclic antidepressants, Bupropion is a relatively weak inhibitor of the neuronal uptake of norepinephrine and dopamine. In addition, Bupropion does not inhibit monoamine oxidase. Bupropion has been found to be essentially inactive at the serotonin transporter (SERT)(IC50 >10 000 nM), however both bupropion and its primary metabolite hydroxybupropion have been found to block the function of cation-selective serotonin type 3A receptors (5-HT3ARs). Bupropion produces dose-related central nervous system (CNS) stimulant effects in animals, as evidenced by increased locomotor activity, increased rates of responding in various schedule-controlled operant behaviour tasks, and, at high doses, induction of mild stereotyped behaviour. Due to these stimulant effects and selective activity at dopamine and norepinephrine receptors, bupropion has been identified as having an abuse potential. Bupropion has a similar structure to the controlled substance [DB01560], and has been identified as having mild amphetamine-like activity, particularly when inhaled or injected. Bupropion is also known to lower the seizure threshold, making any pre-existing seizure conditions a contraindication to its use. This risk is exacerbated when bupropion is combined with other drugs or substances that lower the seizure threshold, such as [cocaine], or in clinical situations that would increase the risk of a seizure such as abrupt alcohol or benzodiazepine withdrawal. As norepinephrine has been shown to have anticonvulsant properties, bupropion's inhibitory effects on NET are thought to contribute to its pro-convulsant activity. Bupropion has been shown to increase blood pressure and pose a risk for exacerbation of unmanaged or pre-existing hypertension, however, clinical trials of bupropion in smokers with CVD have not identified an increased incidence of CV events including stroke or heart attack. In clinical trials, the mean increase in systolic blood pressure associated with the use of bupropion was found to be 1.3 mmHg. |
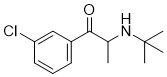
| 分子式 |
C13H18NOCL
|
|---|---|
| 分子量 |
239.74112
|
| 精确质量 |
239.108
|
| CAS号 |
34911-55-2
|
| 相关CAS号 |
Bupropion hydrochloride;31677-93-7;Bupropion hydrobromide;905818-69-1
|
| PubChem CID |
444
|
| 外观&性状 |
Pale yellow oil
|
| 密度 |
1.066g/cm3
|
| 沸点 |
334.8ºC at 760mmHg
|
| 熔点 |
233-234°C
|
| 闪点 |
156.3ºC
|
| LogP |
3.69
|
| tPSA |
29.1
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
16
|
| 分子复杂度/Complexity |
247
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CC(C(=O)C1=CC(=CC=C1)Cl)NC(C)(C)C
|
| InChi Key |
SNPPWIUOZRMYNY-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C13H18ClNO/c1-9(15-13(2,3)4)12(16)10-6-5-7-11(14)8-10/h5-9,15H,1-4H3
|
| 化学名 |
2-(tert-butylamino)-1-(3-chlorophenyl)propan-1-one
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.1712 mL | 20.8559 mL | 41.7119 mL | |
| 5 mM | 0.8342 mL | 4.1712 mL | 8.3424 mL | |
| 10 mM | 0.4171 mL | 2.0856 mL | 4.1712 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。