| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Dipeptidyl peptidase 4 (DPP-4)
|
|---|---|
| 体外研究 (In Vitro) |
在 100 μM CXCR4 阻滞剂或 Src 氧化处理后 30 分钟,暴露于含氧量正常和 H/R 条件的人内皮细胞会发生 Src [Tyr 416] 和 VE-钙粘蛋白 [Tyr731] 的磷酸化。它会氧化并分解 CXCR4(SDF-1α 受体)发泡剂或 Src 偶联的内皮细胞与内皮细胞连接 [1]。
DPP4抑制剂破坏内皮细胞的完整性并增加其通透性[1] 为了测试DPP4抑制剂是否会增加血管通透性,我们用Diprotin A(DipA;Ile-Pro-Ile)处理hECs,并评估Src和VE-cadherin的磷酸化。hECs上的H/R诱导了Src[Tyr 416]和VE-caadherin[Tyr731]的磷酸化(图3a-c)。DPP4抑制剂进一步增加Src[Tyr416]和VE-cadherin[Tyr731]的磷酸化。为了研究DPP4抑制剂对VE钙粘蛋白[Tyr731]的磷酸化是否由SDF-1α/CXCR4配体/受体相互作用和下游信号分子Src激酶介导,我们评估了CXCR4阻断剂(AMD3100)和Src抑制剂(PP2)对Src和VE钙粘素磷酸化的抑制作用。CXCR4阻断剂和Src抑制剂都降低了DPP4抑制剂诱导的人胚胎干细胞中Src[Tyr416]和VE-cadherin[Tyr731]的磷酸化(图3a-c)。然后,我们对人胚胎干细胞中的VE-cadherin进行了免疫荧光染色,以确定DPP4抑制剂诱导的VE-caadherin磷酸化是否真的导致内皮完整性的破坏。DPP4抑制剂破坏了用VE-cadherin标记的内皮细胞间连接。CXCR4阻断剂或Src抑制剂可以阻止这种连接的破坏(图3d,e)。我们使用体外经孔内皮通透性测定法,逐步评估了免疫荧光图像上内皮细胞间连接中断与内皮单层“实际泄漏”的相关性。通过测量FITC葡聚糖(异硫氰酸荧光素偶联葡聚糖;40 kDa)从上腔到下腔通过内皮单层的通道(图3f;详细实验方案见补充图S3)。在上腔中加入DPP4抑制剂后,下腔室中代表内皮通透性的FITC葡聚糖含量显著增加,这被CXCR4阻断剂或Src抑制剂治疗所阻止(图3g)。 |
| 体内研究 (In Vivo) |
使用二蛋白 A(腹腔注射;70 μg/kg;每天两次;7 天)会导致大量血管渗漏以及 Src 和 VE-钙粘蛋白的磷酸化增加。总之,SDF-1α/CXCR4/Src 被二蛋白 A 增强。
多剂量Diprotin A(DipA;Ile-Pro-Ile)和西格列汀的内皮通透性 为了探索剂量反应关系,我们测试了多剂量的<强>Diprotin A(DipA;Ile-Pro-Ile)(1、10、100μM)。由于糖尿病患者的DPP4抑制是通过格列汀实现的,我们还研究了西格列汀对Src和VE-cadherin磷酸化以及内皮通透性的影响。使用0.1、1和10μM的西格列汀浓度来反映服用西格列汀25、100和600的人类志愿者的实际血浆浓度 mg,分别为32,DipA和西格列汀以剂量依赖的方式增加Src[Tyr 416]和VE钙粘蛋白[Tyr731]的磷酸化(图5a,b)。内皮通透性也以剂量依赖的方式增加(DipA和西格列汀的p<0.001;图5c)。两组6个可能的成对比较的p值均<0.01。 DPP4抑制剂体内内皮渗漏[1] 为了评估DPP4抑制剂是否会增加体内血管通透性,我们使用小鼠耳朵进行了Miles通透性测定(补充图S4)。注射SDF-1α的耳朵因全身给药的伊文思蓝染料外渗而变蓝(图6a,b)。腹膜内注射DPP4抑制剂(Diprotin A(DipA;Ile-Pro-Ile))5天后,SDF-1α引起的渗漏加重。然而,CXCR4阻断剂(AMD3100)或Src抑制剂(PP2)显著减少了DPP4抑制剂和SDF-1α的血管渗漏(图6a,b)。 DPP4抑制剂引起的视网膜毛细血管渗漏:早产儿视网膜病变模型[1] 我们使用早产小鼠视网膜病变模型测试了DPP4抑制剂对视网膜血管通透性的体内影响。如实验方案(图7a)所示,新生小鼠幼崽从第7天到第12天暴露于高氧(75%)(高氧情况),然后恢复到正常空气(20%)中5天(相对缺氧情况)。在采集眼睛之前,系统地施用了两种不同的染料;用于血管检查的TRITC偶联的简单斑叶菌凝集素(BS-1凝集素)和用于血管渗漏检查的FITC葡聚糖。与高氧下的新生儿或出生后第12天视网膜相比,出生后第17天小鼠在相对缺氧下的视网膜新生血管增加(补充图S5)。DPP4抑制剂(Diprotin A(DipA;Ile-Pro-Ile))的全身给药不仅增加了血管(补充图S6),还增加了血管渗漏(图7b-d),而CXCR4阻断剂(AMD3100)可以预防血管渗漏。 |
| 酶活实验 |
体外渗透性测定[1]
FITC-葡聚糖(40kDa)是一种易于检测的示踪剂。通过FITC-葡聚糖穿过hECs单层来评估内皮膜的渗透性。实验前两天,将hECs接种到包被纤维连接蛋白的0.4μm孔24孔大小的细胞培养插入物上。将细胞在添加了0.5%胎牛血清的EBM中在标准培养条件下(37°C、95%加湿空气和5%二氧化碳)饥饿培养18小时。在实验开始时,用CXCR4阻断剂(AMD3100;1μg/ml)或Src抑制剂(PP2;1μM)预处理培养基。DPP4抑制剂(Diprotin A(DipA;Ile-Pro-Ile),Ile-Pro-Ile;100μM)在CXCR4阻断剂或Src抑制剂治疗后30分钟施用。DPP4抑制剂处理后60分钟,将浓度为20μg/ml的FITC-葡聚糖加入上腔(Diprotin a(DipA;Ile-Pro-Ile)的药物处理时间:60分钟)。在37°C下孵育20分钟后,从下腔室抽取100μl培养基(渗透时间:20分钟)。为了探索剂量反应关系,在上腔中加入多剂量的<强>Diprotin A(DipA;Ile-Pro-Ile)(1,10,100μM)和西格列汀(0.1,1,10μM)。30分钟后,将FITC葡聚糖加入上腔(药物处理时间:30分钟)。在FITC-葡聚糖处理后5分钟抽取下腔室培养基(渗透时间:5分钟)。通过荧光分光荧光计测定下腔室的荧光。 |
| 细胞实验 |
蛋白质印迹分析[1]
细胞类型: 人内皮细胞[1] 测试浓度: 100 μM 孵育时间: 使用 CXCR4 阻滞剂或 Src 抑制剂治疗后 30 分钟 实验结果: hEC [Tyr731] 中 Src [Tyr 416] 和 VE-钙粘蛋白的磷酸化诱导。 |
| 动物实验 |
Animal/Disease Models: Streptozotocin-induced wild-type C57/BL6 mouse diabetic retinopathy model [1]
Doses: 70 μg/kg Route of Administration: intraperitoneal (ip) injection; /VE-cadherin signaling induces leaky blood vessels [1]. twice (two times) daily; 7 days Experimental Results: Induces vascular leakage by enhancing SDF-1α/CXCR4/Src/VE-cadherin signaling pathway. In-vivo permeability assay [1] Miles assay was performed in wild-type C57/BL6 mice. Three groups of mice received an intra-peritoneal injection of DPP4-inhibitor (Diprotin A (DipA; Ile-Pro-Ile); 70 μg/kg twice daily), and another group received vehicle for 5 days. The specific dose of DPP4-inhibitor used in this study has been shown to mediate therapeutic effects in murine models20,51. After 5 days, 2 groups of mice injected with DPP4-inhibitor received an intra-peritoneal injection of CXCR4-blocker (AMD3100; 7.5 mg/kg, once per day) or Src-inhibitor (PP2; 1 mg/kg, once per day). Thirty minutes later, each mouse was injected with PBS into its right ear and SDF-1α (250 ng) to its left ear, and this was followed by an injection of intra-cardiac 0.5% Evans blue dye. Photographs of the ears were obtained, and the mice were euthanized 30 minutes later. Mouse ear tissue was collected with an 8 mm skin punch and was incubated in 300 μl of formamide at 56 °C for 48 hours. The quantity of Evans blue dye in the tissues and standards was determined by assessing the optical density at 600 nm. Retinopathy of prematurity model [1] The oxygen exposure protocol placed oxygen-exposed mouse pups with their nursing mothers in the same covered plastic box with 75% oxygen from postnatal day 7 through postnatal day 12 as previously described42. The oxygen was delivered at 75 ± 2%, and it was monitored at least three times daily during the oxygen exposure period. Oxygen concentrations were measured with an oxygen monitor. On postnatal day 12, the animals were returned to room air and were subsequently sacrificed by a lethal intra-peritoneal injection of chloral hydrate (360 mg/kg) on postnatal day 17. DPP4-inhibitor injection (Diprotin A (DipA; Ile-Pro-Ile); 70 μg/kg, twice daily) was administered from postnatal day 12 to 17. The control mice were injected with PBS in the same manner as DPP4-inhibitor. CXCR4-blocker (AMD3100; 7.5 mg/kg, once per day) was also injected in the same manner as that for the DPP4-inhibitor. BS-1 Lectin (Sigma-Aldrich, St. Louis, MO, USA) was infused systemically for vascularity examination and FITC-dextran or permeability examination. Both eyes of each animal were used for examination of the retinal vascular pattern after flat mounting of the retina. Streptozotocin-induced diabetic retinopathy model [1] To induce diabetes mellitus, 180 mg/kg of intra-peritoneal STZ was injected into 7-week-old C57/BL6 mice. Blood sugar levels from tail vein blood samples and body weight were monitored weekly. Before the mice were sacrificed, 250 μl of whole blood were drained from the heart for hemoglobin A1c (HbA1c) examination. Two weeks after STZ injection, the mice were confirmed to be diabetic if the BST level was greater than 500 mg/dl. These mice were divided into 4 groups: STZ only; STZ + DPP4-inhibitor; STZ + DPP4-inhibitor + CXCR4-blocker; and STZ + DPP4-inhibitor + Src-inhibitor. Intra-peritoneal DPP4-inhibitor (Diprotin A (DipA; Ile-Pro-Ile); 70 μg/kg twice daily) was injected for 7 days beginning from 6 weeks after STZ injection. Single doses of intra-peritoneal CXCR4-blocker (AMD3100; 7.5 mg/kg, once per day) and Src-inhibitor (PP2; 1 mg/kg, once per day) were also injected in the same manner as that for the DPP4-inhibitor. TRITC-conjugated BS-1 Lectin was infused for vascularity examination and FITC-dextran for permeability examination. Both eyes of each mouse were used for examination of the retinal vascular pattern after flat mounting of the retina. Images were obtained with a Nikon DS-Qi2 CMOS camera head mounted on a Nikon Ti-E motorized inverted microscope. To quantify the fluorescence intensity of FITC-dextran, captured images from each experiment were analyzed using NIS-Elements and ROI statistics software. Using the black-green interface, we created region of interest on each captured image. By ROI statistics, we calculated the mean intensity of FITC-dextran in the region of interest of each captured image. After the retinal vascularity examination, western blot analysis for phosphorylated Src and VE-cadherin was performed using the retinal tissue. |
| 毒性/毒理 (Toxicokinetics/TK) |
mouse LD intravenous >250 mg/kg Journal of Antibiotics., 37(422), 1984 [PMID:6427168]
|
| 参考文献 | |
| 其他信息 |
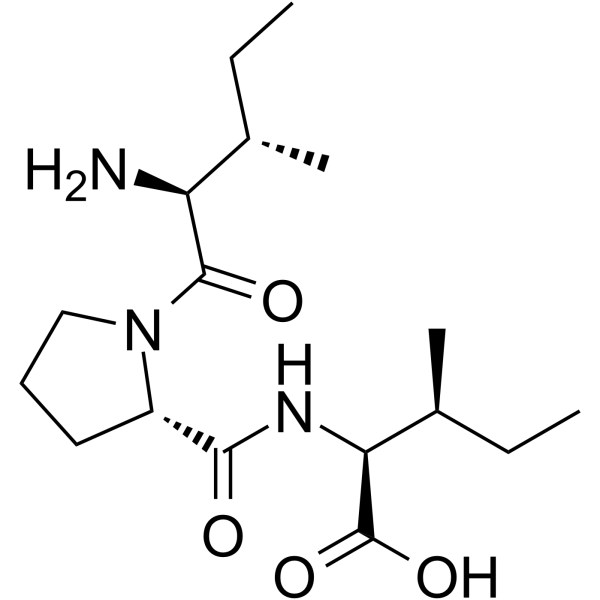
(2S,3S)-2-[[[(2S)-1-[(2S,3S)-2-amino-3-methyl-1-oxopentyl]-2-pyrrolidinyl]-oxomethyl]amino]-3-methylpentanoic acid is a peptide.
diprotin A has been reported in Bacillus cereus with data available. The inhibitors of CD26 (dipeptidyl peptidase-4; DPP4) have been widely prescribed to control glucose level in diabetic patients. DPP4-inhibitors, however, accumulate stromal cell-derived factor-1α (SDF-1α), a well-known inducer of vascular leakage and angiogenesis both of which are fundamental pathophysiology of diabetic retinopathy. The aim of this study was to investigate the effects of DPP4-inhibitors on vascular permeability and diabetic retinopathy. DPP4-inhibitor (Diprotin A (DipA; Ile-Pro-Ile)or sitagliptin) increased the phosphorylation of Src and vascular endothelial-cadherin (VE-cadherin) in human endothelial cells and disrupted endothelial cell-to-cell junctions, which were attenuated by CXCR4 (receptor of SDF-1α)-blocker or Src-inhibitor. Disruption of endothelial cell-to-cell junctions in the immuno-fluorescence images correlated with the actual leakage of the endothelial monolayer in the transwell endothelial permeability assay. In the Miles assay, vascular leakage was observed in the ears into which SDF-1α was injected, and this effect was aggravated by DPP4-inhibitor. In the model of retinopathy of prematurity, DPP4-inhibitor increased not only retinal vascularity but also leakage. Additionally, in the murine diabetic retinopathy model, DPP4-inhibitor increased the phosphorylation of Src and VE-cadherin and aggravated vascular leakage in the retinas. Collectively, DPP4-inhibitor induced vascular leakage by augmenting the SDF-1α/CXCR4/Src/VE-cadherin signaling pathway. These data highlight safety issues associated with the use of DPP4-inhibitors. |
| 分子式 |
C17H31N3O4
|
|---|---|
| 分子量 |
341.44574
|
| 精确质量 |
341.231
|
| 元素分析 |
C, 59.80; H, 9.15; N, 12.31; O, 18.74
|
| CAS号 |
90614-48-5
|
| 相关CAS号 |
Diprotin A TFA;209248-71-5
|
| PubChem CID |
94701
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.14 g/cm3
|
| 沸点 |
583.1ºC at 760 mmHg
|
| 闪点 |
306.5ºC
|
| 折射率 |
1.517
|
| LogP |
1.995
|
| tPSA |
112.73
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
24
|
| 分子复杂度/Complexity |
469
|
| 定义原子立体中心数目 |
5
|
| SMILES |
CC[C@H](C)[C@@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(=O)O)N
|
| InChi Key |
JNTMAZFVYNDPLB-PEDHHIEDSA-N
|
| InChi Code |
InChI=1S/C17H31N3O4/c1-5-10(3)13(18)16(22)20-9-7-8-12(20)15(21)19-14(17(23)24)11(4)6-2/h10-14H,5-9,18H2,1-4H3,(H,19,21)(H,23,24)/t10-,11-,12-,13-,14-/m0/s1
|
| 化学名 |
(2S,3S)-2-[[(2S)-1-[(2S,3S)-2-amino-3-methylpentanoyl]pyrrolidine-2-carbonyl]amino]-3-methylpentanoic acid
|
| 别名 |
diprotin A; 90614-48-5; Ile-Pro-Ile; isoleucylprolylisoleucine; isoleucyl-prolyl-isoleucine; l-isoleucyl-l-prolyl-l-isoleucine; N-(1-L-Isoleucyl-L-prolyl)-L-isoleucine; MFCD00038707;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ≥ 100 mg/mL (~292.87 mM)
DMSO : ~100 mg/mL (~292.87 mM) |
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9287 mL | 14.6434 mL | 29.2869 mL | |
| 5 mM | 0.5857 mL | 2.9287 mL | 5.8574 mL | |
| 10 mM | 0.2929 mL | 1.4643 mL | 2.9287 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。