| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| Other Sizes |
|
| 靶点 |
GLP-1 receptor
|
|---|---|
| 体外研究 (In Vitro) |
Dulaglutide(50 nM 和 100 nM;24 小时)可改善 ox-LDL 诱导的氧化应激并抑制 ox-LDL 诱导的人主动脉内皮细胞 (HAEC) 线粒体功能障碍[1]。细胞活力测定[1] 细胞系:人主动脉内皮细胞 (HAEC) 浓度:50 nM、100 nM 孵育时间:24 小时 结果:抑制 ox-LDL 诱导的细胞活力降低和乳酸脱氢酶 (LDH) 释放。
杜拉鲁肽改善了ox-LDL诱导的氧化应激和线粒体功能障碍。[1] 杜拉鲁肽抑制ox-LDL诱导的IL-1β、IL-6、MCP-1和HMG-1的分泌。[1] 杜拉鲁肽抑制ox-LDL诱导的细胞存活率降低和LDH释放。[1] 杜拉鲁肽通过抑制VCAM-1、E-选择素抑制THP-1与HAEC的附着。[1] 杜拉鲁肽通过抑制p53的激活来促进KLF2的表达。[1] |
| 体内研究 (In Vivo) |
在大鼠致癌性研究中,杜拉鲁肽(0、0.05、0.5、1.5 或 5 mg/kg;皮下注射;每周两次,持续 93 周)会增加甲状腺 C 细胞增生和肿瘤的发生率[2]。动物模型:大鼠和转基因小鼠[1] 剂量:0、0.05、0.5、1.5或5 mg/kg; 0、0.3、1 或 3 mg/kg 给药:皮下注射,每周两次,持续 93 周; SC,每周两次,持续 26 周 结果:弥漫性 C 细胞增生和腺瘤在统计学上增加,>0.5 mg/kg。随着时间的推移,小鼠的全身暴露量减少。
在大鼠和转基因小鼠中评估杜拉鲁肽的致瘤潜力。大鼠每周皮下注射两次杜拉鲁肽0、0.05、0.5、1.5或5mg/kg,持续93周,分别相当于基于血浆曲线下面积的最大推荐人体剂量的0、0.5、7、20和58倍。转基因小鼠每周两次皮下注射杜拉鲁肽0、0.3、1或3mg/kg,持续26周。杜拉鲁肽的作用仅限于甲状腺C细胞。在大鼠中,0.5 mg/kg或更高剂量的弥漫性C细胞增生和腺瘤在统计学上增加(5 mg/kg时P≤.01),5 mg/kg时C细胞癌在数量上增加。与对照组相比,0.5、1.5和5 mg/kg的雌性大鼠局灶性C细胞增殖更高。在转基因小鼠中,在任何剂量下都没有观察到杜拉鲁肽相关的C细胞增生或肿瘤;然而,在所有杜拉鲁肽组中都观察到C细胞的最小细胞质肥大。随着时间的推移,小鼠的全身暴露量逐渐减少,这可能是由于抗药物抗体反应。在一项为期52周的研究中,旨在定量C细胞质量和血浆降钙素反应,大鼠每周两次皮下注射杜拉鲁肽0或5mg/kg。杜拉鲁肽增加了局灶性C细胞增生;C细胞质量没有发生定量增加。与C细胞质量缺乏形态计量学变化一致,杜拉鲁肽不影响弥漫性C细胞增生或基础或钙刺激的血浆降钙素的发生率,这表明在大鼠致癌性研究的最初52周内没有发生C细胞质量的弥漫性增加[3]。 |
| 酶活实验 |
活性氧(ROS)的评估[1]
使用2′,7′-二氯二氢荧光素二乙酸酯(DCFH-DA)染色测量HAEC中的细胞内ROS。在有或没有浓度为50和100 nM的dulaglutide/杜拉鲁肽的情况下,用ox-LDL(100μg/ml)刺激HAEC 24小时,并用PBS洗涤3次。然后在37°C的黑暗中用5μM DCFH-DA加载细胞15分钟。使用蔡司荧光显微镜观察荧光信号。使用Image J软件计算细胞内ROS。简而言之,在荧光图像中定义了感兴趣区域(ROI),并计算了所定义ROI中存在的细胞的平均数量。计算ROI中的积分密度值(IDV),并除以平均细胞数。结果用于表示细胞内ROS的平均水平。 还原型谷胱甘肽(GSH)测定[1] 使用荧光测定法测定HAEC中还原型谷胱甘肽(GSH)的细胞内水平。在有或没有浓度为50和100 nM的dulaglutide/杜拉鲁肽的情况下,用ox-LDL(100μg/ml)刺激HAEC 24小时。然后将细胞收集在冰冷的5%偏磷酸(MPA)中。然后对细胞进行超声处理,并在14000×g下离心5分钟。上清液与等体积的OPAME在甲醇和硼酸盐缓冲液中孵育,并在室温下孵育15分钟。在350 nm激发和420 nm发射下记录荧光信号。 线粒体膜电位(MMP)的测定[1] 使用四甲基罗丹明甲酯(TMRM)染色测定HAEC中MMP的细胞内水平。在存在或不存在浓度为50和100 nM的dulaglutide的情况下,用ox-LDL(100μg/ml)刺激HAEC 24小时。然后用PBS洗涤细胞3次,用20 nmol/L TMRM探测。在37°C下孵育1小时后,将细胞洗涤3次,并使用蔡司荧光显微镜观察荧光信号。 |
| 细胞实验 |
细胞系:人主动脉内皮细胞 (HAEC)
浓度:50 nM、100 nM 孵育时间:24 小时 结果:抑制 ox-LDL 诱导的细胞活力降低和乳酸脱氢酶 (LDH) 释放)。 细胞粘附试验[1] 将HAEC培养至80%融合。在存在或不存在浓度为50和100 nM的杜拉鲁肽的情况下,用ox-LDL(100μg/ml)刺激细胞24小时。共有2×105个THP-1单核细胞用钙黄绿素乙酰氧基甲酯染色30分钟,并与HAEC孵育2小时。将未附着的THP-1细胞洗掉,并用荧光显微镜观察附着的THP-1细胞。 细胞活力评估[1] 将HAEC接种到6孔板中,在有或没有浓度为50和100 nM的<强>杜拉鲁肽的情况下,用ox-LDL(100μg/ml)刺激24小时。经过3次温和洗涤后,加入终浓度为5 mg/ml的3-(4,5-二甲基噻唑-2-基)-2,5-二苯基溴化四氮唑(MTT)的无酚红培养基,在37°C的黑暗中孵育4小时,产物用二甲亚砜(DMSO)溶解。测量570 nm处的OD值以反映存活率百分比。 乳酸脱氢酶(LDH)释放的测定[1] 将HAEC接种到6孔板中,在有或没有浓度为50和100 nM的杜拉鲁肽的情况下,用ox-LDL(100μg/ml)刺激24小时。收集50μl上清液,并在新鲜的96孔板中与50μl LDH测定试剂混合。在黑暗中孵育30分钟后,用50μl停止缓冲液停止反应。记录490 nm处的OD值以评估LDH释放。 |
| 动物实验 |
Rats and Transgenic mice
0, 0.05, 0.5, 1.5, or 5 mg/kg; 0, 0.3, 1, or 3 mg/kg SC, twice week, for 93 weeks; SC, twice week, for 26 weeks Plasma dulaglutide toxicokinetics [3] Twenty-six-week mouse study [3] Blood samples were collected on days 1, 85, and 176 of the dosing phase. Blood was collected from three animals per sex per group per time point. Blood was drawn before dosing and 4, 12, 24, 48, and 96 hours after dosing on days 1 and 176 and before dosing and 24 hours after dosing on day 85. Plasma samples were analyzed for dulaglutide concentrations using a validated ELISA method. Microtiter plates were coated with mouse antihuman IgG (Fc) antibody. Dulaglutide standards, controls, and samples were prepared in mouse plasma. After the preparation, the samples were incubated on the coated plates for approximately 1.5 hours at room temperature. The dulaglutide complex on the plate was bound with a guinea pig anti-GLP-1 active antiserum and then detected using a goat anti-guinea pig IgG-horseradish peroxidase with tetramethyl benzidine substrate. The standard curve ranged from 0.25 to 125 ng/mL, with 2.0 and 30 ng/mL being the lower and upper limits of quantitation, respectively. Ninety-three-week carcinogenicity per 52-week rat thyroid C-cell studies [3] For the 93-week carcinogenicity study, blood was drawn (three rats per sex per group per time point) before dosing and 4, 12, 24, 48, and 96 hours after dosing on days 1 and 24 and before dosing and 24 hours after dosing at weeks 13, 26, 78, and 93. For the 52-week morphometry study, blood samples were collected (three rats per sex per group per time point) approximately 6 days after dosing at the time the animals were killed on days 94, 185, 276, and 374. Plasma samples for both studies were analyzed for dulaglutide concentrations using a validated ELISA method. Microtiter plates were coated with monoclonal anti-GLP-1 antibody. Dulaglutide standards, controls, and samples were prepared in rat plasma. After the preparation, the samples were incubated on the coated plates for approximately 1.5 hours at room temperature. The dulaglutide complex on the plate was detected using a mouse antihuman IgG4-horseradish peroxidase antibody (Southern Biotech) with tetramethyl benzidine substrate. The standard curve ranged from 0.39 to 50 ng/mL, with 0.80 and 40 ng/mL being the lower and upper limits of quantitation, respectively. Fifty-two-week rat thyroid C-cell study [3] Microtiter plates were coated with mouse antihuman IgG (Fc) antibody. Dulaglutide standards, controls, and samples were prepared in rat plasma. After the preparation, the samples were incubated on the coated plates for approximately 1 hour at room temperature. The dulaglutide complex on the plate was bound with a mouse IgG2a kappa anti-GLP-1 antibody and then detected using a goat antimouse IgG2a-horseradish peroxidase with tetramethyl benzidine substrate. The standard curve ranged from 0.40 to 100 ng/mL, with 0.80 and 40 ng/mL being the lower and upper limits of quantitation, respectively. |
| 毒性/毒理 (Toxicokinetics/TK) |
Twenty-six-week mouse study [3]
In-life phase [3] Administration of dulaglutide had no effect on survival. No dulaglutide-related clinical signs were observed. Mean food consumption for males generally decreased for dulaglutide-treated mice compared with controls, resulting in correlative decreases in mean body weight (Supplemental Figures 1–4). Similar, but generally less prominent effects on food consumption were observed for the treated females but did not produce reductions in growth. Plasma dulaglutide toxicokinetics [3] Times to peak plasma concentration of dulaglutide were observed between 4 and 12 hours after dosing. Exposure to dulaglutide, as assessed by area under the curve (AUC) concentration and peak plasma concentration (Cmax) values, increased with increasing doses but was generally less than proportional with an increasing dose on day 176. Systemic exposure of dulaglutide was similar between males and females (Table 1). Plasma concentrations of dulaglutide on day 85 (not shown) and Cmax and AUC values on day 176 were generally 0.5-fold or less than the corresponding day 1 values (Table 1). The decreases in exposure over the duration of the study are likely due to dulaglutide antidrug antibody (ADA) formation; however, the specific determination of dulaglutide ADA was not conducted. Anatomic pathology [3] There were no detectable dulaglutide-related effects and no evidence of thyroid C-cell hyperplasia or neoplasia in the control or treated groups using routine hematoxylin and eosin-stained sections of the thyroid. In sections of thyroid stained with calcitonin, an increased C-cell cytoplasmic volume was detected in all treated groups given the test article and was recorded as C cells, cytoplasmic hypertrophy/increased calcitonin staining (Table 2). The severity was mild, and there was no qualitative increase in the number of thyroid C cells in dulaglutide-treated mice. Increased mortality and increased incidences of bronchiolar alveolar adenoma and carcinoma, squamous cell papilloma and carcinoma, and hemangioma and hemangiosarcoma were observed in the MNU-positive control animals, reflecting a typical response in this strain of mouse after MNU administration. Ninety-three-week rat carcinogenicity study [3] In-life phase [3] Due to the low survival in the control animals (<20 animals/sex), the study was terminated during week 93 with the agreement of the Executive Carcinogenesis Assessment Committee of the FDA. However, sufficient numbers of animals survived to week 80 to adequately evaluate carcinogenicity. No specific dulaglutide-related cause of death was identified. Survival was numerically increased for both genders in all dulaglutide treatment groups and reached statistical significance (P ≤ .05) in the 0.5- and 5-mg/kg males and in the 0.05-, 0.5-, and 1.5-mg/kg females (Table 3). Compound-related decreases in mean body weight and mean food consumption compared with controls were generally dose dependent (Supplemental Figures 5–8). Plasma dulaglutide toxicokinetics [3] Mean times to peak plasma concentration values of dulaglutide were observed at 12 hours after dosing on day 1 and ranged between 12 and 48 hours at week 52. Exposure to dulaglutide, as assessed by AUC and Cmax values, increased with increasing dose from 0.05 to 5 mg/kg on all days evaluated (Table 1). Peak concentration (Cmax) and AUC0–96h values on day 1 and at week 52 were approximately dose proportional. The exposures were similar in male and female rats at all dose groups and days evaluated. Steady state appeared to have been achieved by week 13, and exposure was maintained through week 93. Values for Cmax and AUC0–96h were higher at week 52 than at day 1, indicating possible accumulation of dulaglutide in rat plasma after multiple dosing for 92 weeks. In addition, mean plasma dulaglutide concentrations for the toxicity groups at the time of week 93 study termination were generally similar to the mean concentrations observed for the respective toxicokinetic groups. Anatomic pathology [3] The incidence of thyroid C-cell adenoma was significantly (P ≤ .05) increased compared with controls in males and females at the dulaglutide 0.5-, 1.5-, and 5-mg/kg doses (Table 4). Animals given 5 mg/kg had a numerically higher dulaglutide-related incidence of thyroid C-cell carcinoma that did not reach statistical significance. The incidence of diffuse C-cell hyperplasia was significantly (P ≤ .05) higher compared with controls in males administered dulaglutide 1.5 mg/kg and 5 mg/kg and in females administered dulaglutide 5 mg/kg. The incidence of focal C-cell hyperplasia was higher compared with controls in females treated with dulaglutide 0.5, 1.5, and 5 mg/kg. The incidence of focal C-cell hyperplasia was lower in males treated with dulaglutide 0.5, 1.5, or 5 mg/kg, with a negative trend after the positive trend for an increase in the incidence of thyroid C-cell adenomas (Supplemental Figures 9 and 10). Fifty-two-week rat thyroid C-cell study [3] In-life phase [3] No dulaglutide-related deaths or clinical signs were observed. Three control rats died within several minutes after receiving the CaCl2 dose on day 365 or 372 of the dosing phase. In addition, three control rats and two rats given 5 mg/kg died at unscheduled intervals. The cause of death was a hematopoietic neoplasm in one rat given 5 mg/kg; the cause of death in the other rats was undetermined. Plasma dulaglutide toxicokinetics [3] Plasma dulaglutide concentrations at interim times the animals were killed (6 d after the last dose on d 94, 185, 276, and 374) generally decreased as the study progressed, and there was considerable variability in the concentrations of dulaglutide on day 185. Plasma dulaglutide concentrations 13 days after the final dose (day 374) were below the quantifiable limit for 18 of the 19 animals given 5 mg/kg. One animal had a plasma concentration of 0.91 ng/mL. The decreases in exposure to dulaglutide over the duration of the study were likely due to the formation of antibodies; however, specific determination of dulaglutide ADA was not conducted. Although dulaglutide plasma concentrations generally decreased over the study, effects related to the pharmacology of dulaglutide [decreased food consumption and decreased body weight gain (Supplemental Figures 11 and 12)] were observed throughout the dosing phase, indicating that active dulaglutide was present in the treated animals throughout the study. Anatomical pathology [3] Mean terminal body weights were decreased in treated animals (83%–88% of control means) at all necropsies. Decreased mean absolute thyroid/parathyroid weight (81% of control mean) and thyroid/parathyroid to brain weight ratio (84% of control mean) in treated animals were considered secondary to the decreased body weights. The only microscopic finding considered to be dulaglutide related was an increased incidence and severity of focal or multifocal C-cell hypertrophy/hyperplasia in the thyroids of treated rats after 52 weeks of dosing (Table 5). Focal or multifocal C-cell hypertrophy/hyperplasia in one or two treated animals after 26 or 39 weeks of dosing was not considered dulaglutide related; this lesion is a spontaneous background change in rats, as evidenced by the occurrence in one control animal after 39 weeks of dosing. Focal and multifocal C-cell hypertrophy/hyperplasias were characterized by variably sized nodules of well-differentiated C cells that often had increased cytoplasmic volume (hypertrophy). C cells in these foci were usually less intensely calcitonin immunopositive than surrounding C cells. C-cell neoplasms were identified only in the animals examined after 52 weeks of dosing, occurred at a similarly low incidence in control and treated animals, and were considered unrelated to dulaglutide. |
| 参考文献 |
|
| 其他信息 |
Atherosclerosis is a common comorbidity of type II diabetes and a leading cause of death worldwide. The presence of oxidized low-density lipoprotein (ox-LDL) drives atherogenesis by inducing oxidative stress, mitochondrial dysfunction, expression of proinflammatory cytokines and chemokines including interleukin (IL)-1β, IL-6, and monocyte chemoattractant protein 1 (MCP-1), adhesion molecules including vascular cellular adhesion molecule 1 (VCAM-1) and E-selectin, and downregulating expression of the Krüppel-like factor 2 (KLF2) transcription factor. Importantly, ox-LDL induced the attachment of THP-1 monocytes to endothelial cells. In the present study, we demonstrate for the first time that the specific glucagon-like peptide 1 receptor (GLP-1R) agonist dulaglutide may prevent these atherosclerotic effects of ox-LDL by preventing suppression of KLF2 by p53 protein in human aortic endothelial cells. KLF2 has been shown to play a major role in protecting vascular endothelial cells from damage induced by ox-LDL and oscillatory shear, and therefore, therapies capable of mediating KLF2 signaling may be an attractive treatment option for preventing the development and progression of atherosclerosis. [1]
Background: Three different glucagon-like peptide-1 (GLP-1) receptor agonists reduce cardiovascular outcomes in people with type 2 diabetes at high cardiovascular risk with high glycated haemoglobin A1c (HbA1c) concentrations. We assessed the effect of the GLP-1 receptor agonist dulaglutide on major adverse cardiovascular events when added to the existing antihyperglycaemic regimens of individuals with type 2 diabetes with and without previous cardiovascular disease and a wide range of glycaemic control. Methods: This multicentre, randomised, double-blind, placebo-controlled trial was done at 371 sites in 24 countries. Men and women aged at least 50 years with type 2 diabetes who had either a previous cardiovascular event or cardiovascular risk factors were randomly assigned (1:1) to either weekly subcutaneous injection of dulaglutide (1·5 mg) or placebo. Randomisation was done by a computer-generated random code with stratification by site. All investigators and participants were masked to treatment assignment. Participants were followed up at least every 6 months for incident cardiovascular and other serious clinical outcomes. The primary outcome was the first occurrence of the composite endpoint of non-fatal myocardial infarction, non-fatal stroke, or death from cardiovascular causes (including unknown causes), which was assessed in the intention-to-treat population. This study is registered with ClinicalTrials.gov, number NCT01394952. Findings: Between Aug 18, 2011, and Aug 14, 2013, 9901 participants (mean age 66·2 years [SD 6·5], median HbA1c 7·2% [IQR 6·6-8·1], 4589 [46·3%] women) were enrolled and randomly assigned to receive dulaglutide (n=4949) or placebo (n=4952). During a median follow-up of 5·4 years (IQR 5·1-5·9), the primary composite outcome occurred in 594 (12·0%) participants at an incidence rate of 2·4 per 100 person-years in the dulaglutide group and in 663 (13·4%) participants at an incidence rate of 2·7 per 100 person-years in the placebo group (hazard ratio [HR] 0·88, 95% CI 0·79-0·99; p=0·026). All-cause mortality did not differ between groups (536 [10·8%] in the dulaglutide group vs 592 [12·0%] in the placebo group; HR 0·90, 95% CI 0·80-1·01; p=0·067). 2347 (47·4%) participants assigned to dulaglutide reported a gastrointestinal adverse event during follow-up compared with 1687 (34·1%) participants assigned to placebo (p<0·0001). Interpretation: Dulaglutide could be considered for the management of glycaemic control in middle-aged and older people with type 2 diabetes with either previous cardiovascular disease or cardiovascular risk factors. [2] |
| 精确质量 |
3313.597
|
|---|---|
| CAS号 |
923950-08-7
|
| 相关CAS号 |
GLP-1 moiety from Dulaglutide; 1197810-60-8
|
| PubChem CID |
171042928
|
| 序列 |
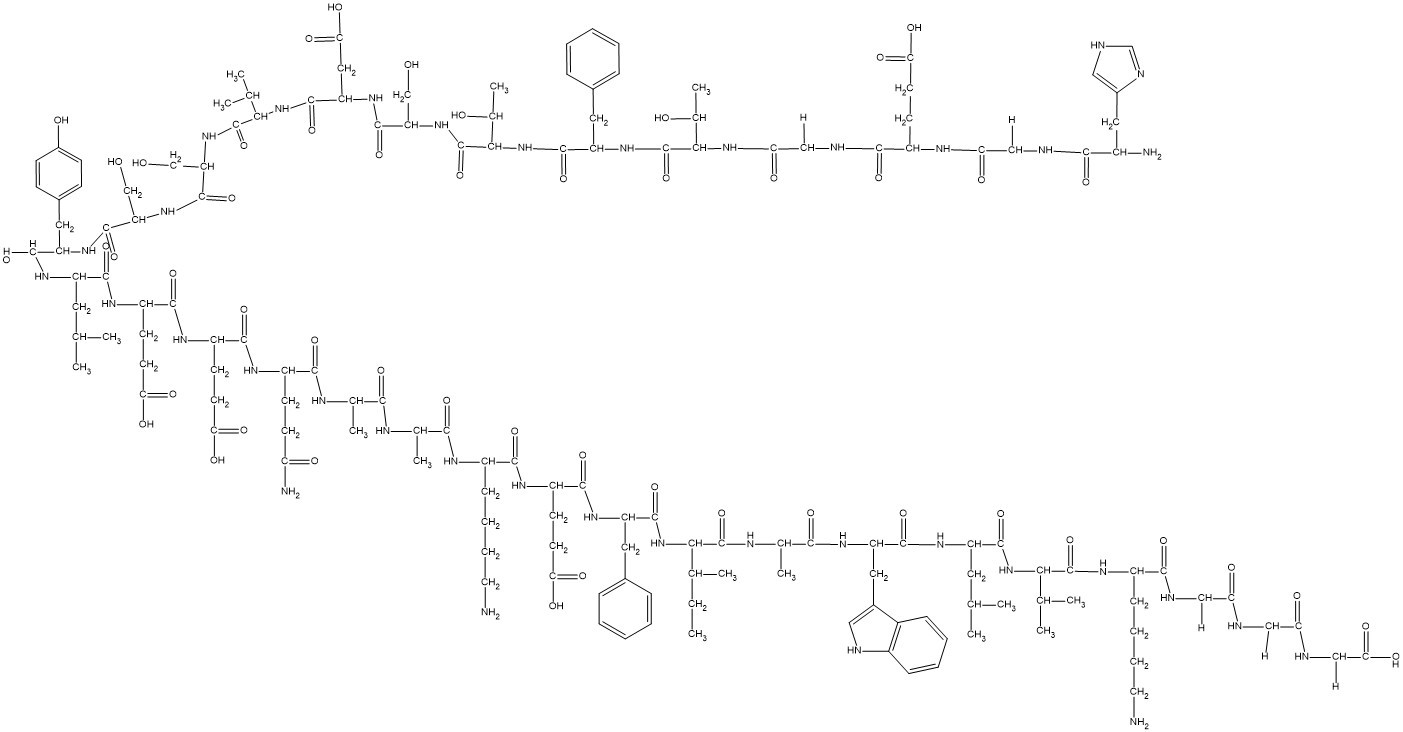
HGEGTFTSDVSSYLEEQAAKEFIAWLVKGGG
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.4±0.1 g/cm3
|
| 折射率 |
1.706
|
| LogP |
3.81
|
| tPSA |
1380
|
| 氢键供体(HBD)数目 |
48
|
| 氢键受体(HBA)数目 |
53
|
| 可旋转键数目(RBC) |
109
|
| 重原子数目 |
235
|
| 分子复杂度/Complexity |
7740
|
| 定义原子立体中心数目 |
29
|
| SMILES |
CC[C@H](C)[C@@H](C(=O)N[C@@H](C)C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)NCC(=O)NCC(=O)O)NC(=O)[C@H](CC3=CC=CC=C3)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC4=CC=C(C=C4)O)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC5=CC=CC=C5)NC(=O)[C@H]([C@@H](C)O)NC(=O)CNC(=O)[C@H](CCC(=O)O)NC(=O)CNC(=O)[C@H](CC6=CNC=N6)N
|
| InChi Key |
HPNPLWNTQBSMAJ-FBXRENMFSA-N
|
| InChi Code |
InChI=1S/C149H221N37O49/c1-16-76(10)121(147(233)164-79(13)126(212)172-103(58-85-61-155-90-34-24-23-33-88(85)90)137(223)174-99(54-73(4)5)138(224)183-119(74(6)7)145(231)171-91(35-25-27-51-150)128(214)159-64-110(195)156-63-109(194)157-67-118(208)209)185-139(225)101(55-82-29-19-17-20-30-82)175-134(220)97(45-50-116(204)205)168-131(217)92(36-26-28-52-151)166-125(211)78(12)162-124(210)77(11)163-130(216)94(41-46-108(153)193)167-132(218)95(43-48-114(200)201)169-133(219)96(44-49-115(202)203)170-135(221)98(53-72(2)3)173-136(222)100(57-84-37-39-87(192)40-38-84)176-142(228)105(68-187)179-144(230)107(70-189)180-146(232)120(75(8)9)184-141(227)104(60-117(206)207)177-143(229)106(69-188)181-149(235)123(81(15)191)186-140(226)102(56-83-31-21-18-22-32-83)178-148(234)122(80(14)190)182-112(197)66-160-129(215)93(42-47-113(198)199)165-111(196)65-158-127(213)89(152)59-86-62-154-71-161-86/h17-24,29-34,37-40,61-62,71-81,89,91-107,119-123,155,187-192H,16,25-28,35-36,41-60,63-70,150-152H2,1-15H3,(H2,153,193)(H,154,161)(H,156,195)(H,157,194)(H,158,213)(H,159,214)(H,160,215)(H,162,210)(H,163,216)(H,164,233)(H,165,196)(H,166,211)(H,167,218)(H,168,217)(H,169,219)(H,170,221)(H,171,231)(H,172,212)(H,173,222)(H,174,223)(H,175,220)(H,176,228)(H,177,229)(H,178,234)(H,179,230)(H,180,232)(H,181,235)(H,182,197)(H,183,224)(H,184,227)(H,185,225)(H,186,226)(H,198,199)(H,200,201)(H,202,203)(H,204,205)(H,206,207)(H,208,209)/t76-,77-,78-,79-,80+,81+,89-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,119-,120-,121-,122-,123-/m0/s1
|
| 化学名 |
(4S)-5-[[2-[[(2S,3R)-1-[[(2S)-1-[[(2S,3R)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-5-amino-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[(2S)-1-[[(2S)-1-[[(2S,3S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[2-[[2-(carboxymethylamino)-2-oxoethyl]amino]-2-oxoethyl]amino]-1-oxohexan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-1-oxopropan-2-yl]amino]-3-methyl-1-oxopentan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-1-oxohexan-2-yl]amino]-1-oxopropan-2-yl]amino]-1-oxopropan-2-yl]amino]-1,5-dioxopentan-2-yl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-3-carboxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxobutan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-3-hydroxy-1-oxobutan-2-yl]amino]-2-oxoethyl]amino]-4-[[2-[[(2S)-2-amino-3-(1H-imidazol-4-yl)propanoyl]amino]acetyl]amino]-5-oxopentanoic acid
|
| 别名 |
Dulaglutide; GLP-1 moiety from Dulaglutide; 923950-08-7; Dulaglutide; 1197810-60-8; HPNPLWNTQBSMAJ-FBXRENMFSA-N; LY2189265
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
Note: 如何溶解多肽产品?请参考本产品网页右上角“产品说明书”文件,第4页。 注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。 注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Perioperative Stress Hyperglycemia in General and Vascular Surgery Patients
CTID: NCT04862234
Phase: Phase 4 Status: Recruiting
Date: 2024-09-20