| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
S1P1 ( IC50 = 1.88 nM )
|
|---|---|
| 体外研究 (In Vitro) |
Etrasimod arginine/APD-334 是一种结构新颖、选择性、功能性的 S1P1 拮抗剂。在表达 HA 标记的 S1P1 的 CHO 细胞中,APD334 的 IC50 值为 1.88 nM。观察到对人 S1P4 和 S1P5 的中等激动作用,但在效力和功效方面相对于 S1P1 有所降低。 APD334 对人 S1P2 和 S1P3 没有任何激动或拮抗作用。 APD334 口服给药后实现了良好的中枢暴露,并在多个临床前物种中具有良好的药代动力学特征。 S1P1 活性在小鼠 (EC50=0.44 nM)、大鼠 (EC50=0.32 nM)、狗 (EC50=0.34 nM) 和猴子 (EC50=0.32 nM) 中保持不变[1]。
|
| 体内研究 (In Vivo) |
Etrasimod 精氨酸/APD-334 在所有物种中具有相对较低的全身清除率(<肝血流量的 4%)和较高的 Cmax。与啮齿动物相比,狗和猴子的分布容积 (Vss) 显着减少。口服生物利用度在 40-100% 范围内,终末期半衰期从猴子的 6 小时到狗的长达 29 小时不等。 siponimod(另一种目前正在进行人体试验的 S1P1 调节剂)的大鼠和猴子 t1/2 值已公开,分别为 6 小时和 19 小时[1]。大鼠:在雄性 Sprague-Dawley 大鼠中测定了 APD334 对血液淋巴细胞减少症的诱导作用。简言之,雄性大鼠口服0.5%甲基纤维素(MC)水溶液中配制的APD334,口服剂量为0(仅载体)、0.03(仅小鼠)、0.1、0.3或1mg/kg。在给药后 0、1、3、5、8、16、24、32、48 和 72 小时收集大鼠血液样本[1]。小鼠:在雄性 BALB/c 小鼠中测定了 APD334 对血液淋巴细胞减少症的诱导作用。简言之,向雄性小鼠给予0(仅载体)、0.03(仅小鼠)、0.1、0.3或1mg/kg口服剂量的在0.5%甲基纤维素(MC)水溶液中配制的APD334。在给药后 0、1、3、5、8、16、24 和 32 小时采集小鼠血液样本[1]。
|
| 动物实验 |
Rats: Male Sprague-Dawley rats are used to assess the effects of APD334 on blood lymphopenia. Male rats are administered an oral dose of APD334 formulated in 0.5% methylcellulose (MC) in water at a rate of 0 mg/kg (vehicle only), 0.03 mg/kg (mice only), 0.1, 0.3, or 1 mg/kg. Samples of rat blood are taken 0, 1, 3, 5, 8, 16, 24, 32, 48, and 72 hours after the dose[1].
Mice: Male BALB/c mice are used to study the effects of APD334 on blood lymphopenia. In summary, APD334 is administered orally to male mice at doses of 0 (vehicle only), 0.03 (mice only), 0.1, 0.3, or 1 mg/kg when formulated in 0.5% methylcellulose (MC) in water. At 0, 1, 3, 5, 8, 16, 24, and 32 hours after administration, mouse blood samples are collected[1]. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Etrasimod mean (SD) steady-state maximum plasma concentration (Cmax) was 113 (27.5) ng/mL and the area under the time concentration curve at the dosing interval (AUCtau) was 2162 (488) ng*h/mL at the recommended dosage. Etrasimod Cmax and AUC are approximately dose-proportional from 0.7 mg to 2 mg (0.35 times up to the recommended dosage). Etrasimod steady state is reached within 7 days with an accumulation of approximately 2- to 3-fold compared to the first dose. The median (range) time to reach etrasimod Cmax (Tmax) is approximately 4 hours (range 2 to 8 hours) after oral administration. No clinically significant differences in the pharmacokinetics of etrasimod were observed following administration of etrasimod with a high-fat meal (800 to 1000 calories). Approximately 82% of the total radioactive etrasimod dose was recovered in the feces and 5% in the urine within 336 hours. Approximately 11% of the administered radioactive dose was excreted as unchanged etrasimod in feces and none was excreted unchanged in urine. The mean apparent volume of distribution of etrasimod is 66 (24) L. The apparent steady-state oral clearance of etrasimod is approximately 1 L/h after oral administration. Metabolism / Metabolites Etrasimod is metabolized by oxidation and dehydrogenation mediated primarily by CYP2C8, CYP2C9, and CYP3A4, with a minor contribution by CYP2C19 and CYP2J2. Etrasimod also undergoes conjugation primarily mediated by UGTs, with a minor contribution by sulfotransferases. Unchanged etrasimod is the main circulating component in plasma. Biological Half-Life The mean plasma elimination half-life (t1/2) of etrasimod is approximately 30 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation No information is available on the use of etrasimod during breastfeeding. Because etrasimod is 98% bound to plasma proteins, the amount in milk is likely to be low. If the mother requires etrasimod, it is not a reason to discontinue breastfeeding. Until more data become available, etrasimod should be used with caution during breastfeeding, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Etrasimod plasma protein binding is 97.9%. |
| 参考文献 | |
| 其他信息 |
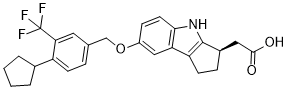
Etrasimod is an organic heterotricyclic compound that is 1,2,3,4-tetrahydrocyclopenta[b]indole substituted by carboxymethyl and [4-cyclopentyl-3-(trifluoromethyl)phenyl]methoxy groups at positions 3R and 7, respectively. It is a potent and functional antagonist of the sphingosine-1-phosphate-1 (S1P1) receptor (IC50 = 1.88 nM in CHO cells). It has a role as an antibacterial agent, a sphingosine-1-phosphate receptor 1 antagonist, an immunosuppressive agent and an anti-inflammatory drug. It is a member of (trifluoromethyl)benzenes, a member of cyclopentanes, an aromatic ether, a monocarboxylic acid and an organic heterotricyclic compound. It is a conjugate acid of an etrasimod(1-).
Etrasimod is a synthetic next-generation selective Sphingosine 1-phosphate (S1P) receptor modulator that targets the S1P1,4,5 with no detectable activity on S1P2 and S1P3 receptors. S1P receptors are membrane-derived lysophospholipid signaling molecules that are involved in the sequestration of circulating peripheral lymphocytes in lymph nodes. Therefore, S1P receptor modulators like etrasimod were investigated in treating immune-mediated diseases like ulcerative colitis where a high level of inflammatory T cells is present in the gastrointestinal tract, thus causing diffuse mucosal inflammation. In fact, it has been observed that antigen-activated T cells within peripheral lymphoid organs can transiently downregulate S1P receptor levels to facilitate immune cells trafficking into the intestinal mucosa. Etrasimod was approved on October 13, 2023, by the FDA under the brand name VELSIPITY for the treatment of adults with moderately to severely active ulcerative colitis. This approval was based on favorable results obtained from Pfizer’s Elevate UC Phase III registrational program, consisting of the Elevate UC 52 and Elevate UC 12 clinical trials, that investigates the efficacy of a 2-mg daily dose regimen of etrasimod, with a 32% and 26% remission rate observed in UC 52 and UC 12 trials respectively. Drug Indication Etrasimod is indicated for the treatment of moderately to severely active ulcerative colitis (UC) in adults. Mechanism of Action Etrasimod is a sphingosine 1-phosphate (S1P) receptor modulator that binds with high affinity to S1P receptors 1, 4, and 5 (S1P1,4,5). Etrasimod has minimal activity on S1P3 (25-fold lower than Cmax at the recommended dose) and no activity on S1P2. Etrasimod partially and reversibly blocks the capacity of lymphocytes to egress from lymphoid organs, reducing the number of lymphocytes in peripheral blood. The mechanism by which etrasimod exerts therapeutic effects in UC is unknown but may involve the reduction of lymphocyte migration into the intestines. Pharmacodynamics Etrasimod causes a reduction in peripheral blood lymphocyte count. In UC-1 and UC-2, mean lymphocyte counts decreased to approximately 50% of baseline at 2 weeks (approximate mean blood lymphocyte counts 0.9 x 109/L) and the lower lymphocyte counts were maintained during treatment with etrasimod. Dose-response relationship analysis indicates there is a dose-dependent reduction in blood lymphocyte counts. After discontinuing etrasimod 2 mg once daily, the median time for peripheral blood lymphocytes to return to the normal range was 2.6 weeks, with approximately 90% of subjects in the normal range within 4.7 weeks. Etrasimod may result in a transient decrease in heart rate and AV conduction upon treatment initiation. In UC-1 and UC-2, the mean (SD) decrease in heart rate was 7.2 (8.98) bpm at 2 to 3 hours after the first dose of etrasimod on Day 1. At 2 times the maximum recommended dose, etrasimod does not cause clinically significant QTc interval prolongation. Reductions in absolute FEV1 were also observed in subjects treated with etrasimod. |
| 分子式 |
C26H26F3NO3
|
|---|---|
| 分子量 |
457.49321
|
| 精确质量 |
457.186
|
| 元素分析 |
C, 68.26; H, 5.73; F, 12.46; N, 3.06; O, 10.49
|
| CAS号 |
1206123-37-6
|
| 相关CAS号 |
1206123-37-6; 1206123-97-8 (arginine)
|
| PubChem CID |
44623998
|
| 外观&性状 |
White to khaki solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
621.4±50.0 °C at 760 mmHg
|
| 闪点 |
329.6±30.1 °C
|
| 蒸汽压 |
0.0±1.9 mmHg at 25°C
|
| 折射率 |
1.606
|
| LogP |
6.43
|
| tPSA |
62.3
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
33
|
| 分子复杂度/Complexity |
695
|
| 定义原子立体中心数目 |
1
|
| SMILES |
FC(F)(F)C1=CC(COC2=CC=C(NC3=C4CC[C@@H]3CC(O)=O)C4=C2)=CC=C1C5CCCC5
|
| InChi Key |
MVGWUTBTXDYMND-QGZVFWFLSA-N
|
| InChi Code |
InChI=1S/C26H26F3NO3/c27-26(28,29)22-11-15(5-8-19(22)16-3-1-2-4-16)14-33-18-7-10-23-21(13-18)20-9-6-17(12-24(31)32)25(20)30-23/h5,7-8,10-11,13,16-17,30H,1-4,6,9,12,14H2,(H,31,32)/t17-/m1/s1
|
| 化学名 |
2-[(3R)-7-[[4-cyclopentyl-3-(trifluoromethyl)phenyl]methoxy]-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl]acetic acid
|
| 别名 |
APD 334; APD334; APD-334; Etrasimod; Velsipity
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ≥ 28 mg/mL (~61.2 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1858 mL | 10.9292 mL | 21.8584 mL | |
| 5 mM | 0.4372 mL | 2.1858 mL | 4.3717 mL | |
| 10 mM | 0.2186 mL | 1.0929 mL | 2.1858 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
A Study to Learn About the Effectiveness of Etrasimod in People With Ulcerative Colitis
CTID: NCT06294925
Phase: Status: Recruiting
Date: 2024-10-28