| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
| 靶点 |
Cell wall synthesis
|
|---|---|
| 体外研究 (In Vitro) |
1. 抗菌活性与协同作用:
- 文献[2]通过肉汤稀释法测定氟氯西林对金黄色葡萄球菌的最低抑菌浓度(MIC)为0.25-2 μg/mL。当与阿莫西林联合使用时,对产β-内酰胺酶菌株的协同作用显著,部分抑菌浓度(FIC)指数≤0.5,显示出相加或协同效应 [2]
|
| 体内研究 (In Vivo) |
氟氯西林的血清消除半衰期为1.31-1.39小时,单次250mg剂量给药后6小时,氟氯西林的血清浓度为0.46微克/mL[1]。口服氟氯西林不会影响汗液电解质,也不是汗液检测的禁忌症[2]。据报道,氟氯西林在人体内代谢为青霉唑酸、具有抗菌活性的5''-羟甲基衍生物和5''-羟甲基衍生物的青霉唑酸。氟氯西林在大鼠体内的代谢相似[3]。
1. 动物模型中的疗效: - 文献[1]在大鼠植入物相关金黄色葡萄球菌感染模型中,比较了氟氯西林(100 mg/kg,每日2次,腹腔注射)与莫西沙星、利福平及联合治疗的效果。结果显示,氟氯西林单药治疗可显著降低植入物周围组织的细菌负荷(p<0.05),但效果弱于利福平联合治疗 [1] 2. 体内协同作用验证: - 文献[2]在小鼠败血症模型中,氟氯西林(50 mg/kg,皮下注射)与阿莫西林(100 mg/kg)联合使用可提高存活率至70%,显著优于单药治疗组(存活率分别为40%和30%) [2] |
| 动物实验 |
Animal Model: Male Wistar rats[1]
Dosage: 200 mg/kg Administration: Intraperitoneal injection; three times/day, for 21 days Result: Reducted germs in the biofilm and in the bone tissue. 1. Implant Infection Model (Reference [1]): - Animals: Male Sprague-Dawley rats (250–300 g) - Infection Induction: Polymethyl methacrylate (PMMA) beads contaminated with Staphylococcus aureus (ATCC 25923) were implanted into the femoral medullary cavity - Dosing Regimen: Treatment was initiated 24 hours after infection. flucloxacillin was dissolved in normal saline at a dose of 100 mg/kg, administered via intraperitoneal injection twice daily for 7 days - Evaluation Indicators: After treatment, the animals were euthanized. Tissue surrounding the implant was collected for bacterial counting and histopathological analysis [1] 2. Sepsis Model (Reference [2]): - Animals: Female BALB/c mice (20–25 g) - Infection Induction: Staphylococcus aureus (1×10⁷ CFU/mouse) was injected via the tail vein - Dosing Regimen: Treatment was initiated 1 hour after infection. flucloxacillin was dissolved in sterile water at a dose of 50 mg/kg, administered via subcutaneous injection three times daily for 3 days - Evaluation Indicators: Survival rates were recorded within 7 days, and blood and organs were collected for bacterial culture [2] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Bioavailability is 50–70% following oral administration. Metabolism / Metabolites Hepatic. Flucloxacillin has known human metabolites that include 6-[[3-(2-Chloro-6-fluorophenyl)-5-(hydroxymethyl)-1,2-oxazole-4-carbonyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid. Biological Half-Life 0.75–1 hour |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Floxacillin (flucloxacillin) is not approved for marketing in the United States by the U.S. Food and Drug Administration. It is acceptable to use during breastfeeding and is frequently used abroad to treat mastitis in nursing mothers. Limited information indicates that floxacillin levels in milk are low and are not expected to cause adverse effects in breastfed infants. Occasionally disruption of the infant's gastrointestinal flora, resulting in diarrhea or thrush have been reported with penicillins, but these effects have not been adequately evaluated. Floxacillin is acceptable in nursing mothers. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. 1. Safety Evaluation (Reference [1]): - Rats in the flucloxacillin treatment group showed no significant weight loss or abnormalities in liver and kidney function (serum ALT, AST, and creatinine levels were not significantly different from those in the control group) [1] 2. Tolerance Observation (Reference [2]): - No adverse reactions such as diarrhea, rash, or neurotoxicity were observed in mice receiving flucloxacillin monotherapy or combination therapy [2] |
| 参考文献 |
|
| 其他信息 |
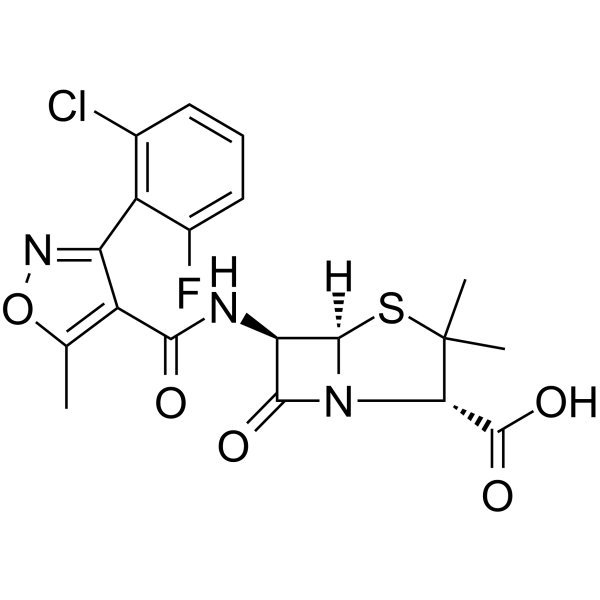
Flucloxacillin is a penicillin compound having a 6beta-[3-(2-chloro-6-fluorophenyl)-5-methyl-1,2-oxazole-4-carboxamido] side-chain. It has a role as an antibacterial drug. It is a penicillin and a penicillin allergen. It is a conjugate acid of a flucloxacillin(1-).
Antibiotic analog of [cloxacillin]. Flucloxacillin is a narrow-spectrum, semisynthetic isoxazolyl penicillin with antibacterial activity. Floxacillin binds to and inactivates penicillin-binding proteins (PBPs) located on the inner membrane of the bacterial cell wall. Inactivation of PBPs interferes with the cross-linkage of peptidoglycan chains necessary for bacterial cell wall strength and rigidity. This interrupts bacterial cell wall synthesis and results in the weakening of the bacterial cell wall, eventually causing cell lysis. Antibiotic analog of CLOXACILLIN. Drug Indication Used to treat bacterial infection by susceptible microorganisms. Mechanism of Action By binding to specific penicillin-binding proteins (PBPs) located inside the bacterial cell wall, flucloxacillin inhibits the third and last stage of bacterial cell wall synthesis. Cell lysis is then mediated by bacterial cell wall autolytic enzymes such as autolysins; it is possible that flucloxacillin interferes with an autolysin inhibitor. 1. Clinical Application Background: - flucloxacillin is a semisynthetic penicillin-class antibiotic. It exerts bactericidal effects by inhibiting bacterial cell wall synthesis and exhibits high activity against β-lactamase-producing staphylococci and streptococci [1][2] 2. Advantages of Combination Therapy: - Reference [2] points out that the combination of flucloxacillin and amoxicillin can expand the antibacterial spectrum and reduce the selective pressure for drug-resistant mutants, making it particularly suitable for mixed infections or severe infections [2] 3. Limitations: - Reference [1] emphasizes that flucloxacillin is ineffective against methicillin-resistant Staphylococcus aureus (MRSA), and treatment regimens should be selected based on drug susceptibility testing [1] |
| 分子式 |
C19H17N3O5FSCL
|
|---|---|
| 分子量 |
453.87178
|
| 精确质量 |
453.056
|
| 元素分析 |
C, 50.28; H, 3.78; Cl, 7.81; F, 4.19; N, 9.26; O, 17.63; S, 7.06
|
| CAS号 |
5250-39-5
|
| 相关CAS号 |
Flucloxacillin sodium;1847-24-1
|
| PubChem CID |
21319
|
| 外观&性状 |
Solid powder
|
| 密度 |
1.6±0.1 g/cm3
|
| 沸点 |
677.3±55.0 °C at 760 mmHg
|
| 闪点 |
363.4±31.5 °C
|
| 蒸汽压 |
0.0±2.2 mmHg at 25°C
|
| 折射率 |
1.672
|
| LogP |
2.6
|
| tPSA |
138.04
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
30
|
| 分子复杂度/Complexity |
758
|
| 定义原子立体中心数目 |
3
|
| SMILES |
O=C([C@@H](C(C)(C)S[C@]1([H])[C@@H]2NC(C3=C(C)ON=C3C4=C(F)C=CC=C4Cl)=O)N1C2=O)O
|
| InChi Key |
UIOFUWFRIANQPC-JKIFEVAISA-N
|
| InChi Code |
InChI=1S/C19H17ClFN3O5S/c1-7-10(12(23-29-7)11-8(20)5-4-6-9(11)21)15(25)22-13-16(26)24-14(18(27)28)19(2,3)30-17(13)24/h4-6,13-14,17H,1-3H3,(H,22,25)(H,27,28)/t13-,14+,17-/m1/s1
|
| 化学名 |
4-Thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid, 6-(((3-(2-chloro-6-fluorophenyl)-5-methyl-4-isoxazolyl)carbonyl)amino)-3,3-dimethyl-7-oxo-, (2S(2alpha,5alpha,6beta))
|
| 别名 |
Floxapen; Flucloxacillin; FLOXACILLIN; 5250-39-5; flucloxacilina; Flucloxacilline; Flucloxacillinum; BRL 2039; 3-(2-Chloro-6-fluorophenyl)-5-methyl-4-isoxazolylpenicillin; BRL 2039; Floxacillin
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2033 mL | 11.0164 mL | 22.0327 mL | |
| 5 mM | 0.4407 mL | 2.2033 mL | 4.4065 mL | |
| 10 mM | 0.2203 mL | 1.1016 mL | 2.2033 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。