| 规格 | 价格 | ||
|---|---|---|---|
| 500mg | |||
| 1g | |||
| Other Sizes |

| 靶点 |
D2 Receptor
|
|---|---|
| 体外研究 (In Vitro) |
氟哌啶醇是一种由中心哌啶结构和N-连接的对氟丁苯部分组成的化合物,中心哌啶结构在4位具有羟基和对氯苯基取代基。它具有5-羟色胺能拮抗剂、第一代抗精神病药、多巴胺能拮抗剂和止吐药的作用。它是一种羟基哌啶、有机氟化合物、芳香酮、叔醇和一氯苯类化合物。
|
| 体内研究 (In Vivo) |
单次动脉内注射氟哌啶醇(1 毫克)可减少多巴胺引发的胰腺分泌。 10 μg 多巴胺在狗胰腺中的作用被 3 mg 氟哌啶醇完全抑制 [1]。在 7 至 10 分钟内,注射 50 mg/kg (2 µc) 麦司卡林 45 分钟后,氟哌啶醇 (10 mg/kg) 和氯丙嗪 (CPZ,15 mg/kg) 会抑制注射。偏离麦司卡林会导致行为改变。氟哌啶醇不影响麦司卡林的消除[2]。
|
| 动物实验 |
In an open, double-blind study of phencyclidine intoxication, 21 white male subjects were later found to have instead ingested ketamine. These subjects were divided into two cohorts, one treated with 5 mg intramuscular haloperidol and the second with an active placebo. Assessment with the Brief Psychiatric Rating Scale revealed significant reduction in symptoms with haloperidol.[2]
|
| 药代性质 (ADME/PK) |
Absorption
Haloperidol is a highly lipophilic compound and is extensively metabolized in humans, which may cause a large interindividual variability in its pharmacokinetics. Studies have found a wide variance in pharmacokinetic values for orally administered haloperidol with 1.7-6.1 hours reported for time to peak plasma concentration (tmax), 14.5-36.7 hours reported for half-life (t1⁄2), and 43.73 μg/L•h [range 14.89-120.96 μg/L•h] reported for AUC. Haloperidol is well-absorbed from the gastrointestinal tract when ingested orally, however, the first-pass hepatic metabolism decreases its oral bioavailability to 40 - 75%. After intramuscular administration, the time to peak plasma concentration (tmax) is 20 minutes in healthy individuals or 33.8 minutes in patients with schizophrenia, with a mean half-life of 20.7 hours. Bioavailability following intramuscular administration is higher than that for oral administration. Administration of haloperidol decanoate (the depot form of haloperidol for long-term treatment) in sesame oil results in slow release of the drug for long-term effects. The plasma concentrations of haloperidol gradually rise, reaching its peak concentration at about 6 days after the injection, with an apparent half-life of about 21 days. Steady-state plasma concentrations are achieved after the third or fourth dose. Route of Elimination In radiolabeling studies, approximately 30% of the radioactivity is excreted in the urine following a single oral administration of 14C-labelled haloperidol, while 18% is excreted in the urine as haloperidol glucuronide, demonstrating that haloperidol glucuronide is a major metabolite in the urine as well as in plasma in humans. Volume of Distribution The apparent volume of distribution was found to range from 9.5-21.7 L/kg. This high volume of distribution is in accordance with its lipophilicity, which also suggests free movement through various tissues including the blood-brain barrier. Clearance Following intravenous administration, the plasma or serum clearance (CL) was found to be 0.39-0.708 L/h/kg (6.5 to 11.8 ml/min/kg). Following oral administration, clearance was found to be 141.65 L/h (range 41.34 to 335.80 L/h). Haloperidol clearance after extravascular administration ranges from 0.9-1.5 l/h/kg, however this rate is reduced in poor metabolizers of C_YP2D6_ enzyme. Reduced CYP2D6 enzyme activity may result in increased concentrations of haloperidol. The inter-subject variability (coefficient of variation, %) in haloperidol clearance was estimated to be 44% in a population pharmacokinetic analysis in patients with schizophrenia. Genetic polymorphism of CYP2D6 has been demonstrated to be an important source of inter-patient variability in the pharmacokinetics of haloperidol and may affect therapeutic response and incidence of adverse effects. Haloperidol is well absorbed from the gastrointestinal tract but first-pass hepatic metabolism decreases oral bioavailability to 40 to 75%. Serum concentration peaks 0.5 to 4 hours after an oral dose. The apparent volume of distribution is about 20 L/kg, consistent with the high lipophilicity of the drug. Haloperidol circulates in blood bound predominantly (90-94%) to plasma proteins. Following administration of haloperidol in animals, the drug is distributed mainly into the liver, with lower concentrations being distributed into the brain, lung, kidneys, spleen, and heart. ... Haloperidol is about 92% bound to plasma proteins. View More
Metabolism / Metabolites
Haloperidol is extensively metabolised in the liver with only about 1% of the administered dose excreted unchanged in urine. In humans, haloperidol is biotransformed to various metabolites, including p-fluorobenzoylpropionic acid, 4-(4-chlorophenyl)-4-hydroxypiperidine, reduced haloperidol, pyridinium metabolites, and haloperidol glucuronide. In psychiatric patients treated regularly with haloperidol, the concentration of haloperidol glucuronide in plasma is the highest among the metabolites, followed, in rank order, by unchanged haloperidol, reduced haloperidol and reduced haloperidol glucuronide. The drug is thought to be metabolized primarily by oxidative N-dealkylation of the piperidine nitrogen to form fluorophenylcarbonic acids and piperidine metabolites (which appear to be inactive), and by reduction of the butyrophenone carbonyl to the carbinol, forming _hydroxyhaloperidol_. The enzymes involved in the biotransformation of haloperidol include cytochrome P450 (CYP) including CYP3A4 and CYP2D6, carbonyl reductase and uridine di-phosphoglucose glucuronosyltransferase enzymes. The greatest proportion of the intrinsic hepatic clearance of haloperidol is performed by glucuronidation and followed by the reduction of haloperidol to reduced haloperidol and by CYP-mediated oxidation. In studies of cytochrome-mediated disposition in vitro, CYP3A4 appears to be the major isoform of the enzyme responsible for the metabolism of haloperidol in humans. The intrinsic clearance of the back-oxidation of reduced haloperidol to the parent compound, oxidative N-dealkylation and pyridinium formation are of the same order of magnitude. This suggests that the same enzyme system is responsible for the above three metabolic reactions. In vivo human studies on haloperidol metabolism have shown that the glucuronidation of haloperidol accounts for 50 to 60% of haloperidol biotransformation and that approximately 23% of the biotransformation was accounted for by the reduction pathway. The remaining 20 to 30% ofthe biotransformation of haloperidol would be via N-dealkylation and pyridinium formation.
Biological Half-Life Following oral administration, the half-life was found to be 14.5-36.7 hours. Following intramuscular injection, mean half-life was found to be 20.7 hours. 10 MG Haloperidol IV and oral administration to healthy volunteers: serum T1/2 10-19 hr after IV and 12-38.3 hr after oral administration. Bioavailability in the order of 60%; distribution volume around 1300 L. PMID:822989 Haloperidol, Elimination: Oral: 24 hours (range 12 to 37 hours). Intramuscular: 21 hours (range, 17 to 25 hours). Intravenous: 14 hours (range, 10 to 19 hours). Haloperidol decanoate, Elimination: Approximately 3 weeks (single or multiple doses). |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION: Haloperidol is an antipsychotic agent. Haloperidol is a synthetic product. Haloperidol is the first of the butyrophenone series of major tranquillizers. Haloperidol is indicated for use in the management of manifestations of psychotic disorders such as schizophrenia and mania. It is indicated for the control of tics and vocal utterances of Tourette's Disorder in children and adults. It is effective for the treatment of severe behaviour problems in children of combative, explosive hyperexcitability. It is also used in the management of Gilles de La Tourette's syndrome, intractable hiccup and as an anti-emetic. HUMAN EXPOSURE: Main risks and target organs: The main features of severe overdosage are extrapyramidal reactions, hypotension, respiratory difficulty and impairment of consciousness. Haloperidol acts mainly as a dopamine antagonist. Summary of clinical effects: Consciousness may be depressed, progressing to coma; paradoxically, some patients manifest confusion, excitement and restlessness. Tremor or muscle twitching, muscle spasm, rigidity and convulsions are seen. Extrapyramidal signs can include dystonia, sometimes severe enough to impair swallowing or breathing; torticollis, oculogyric crises and opisthotonos. The pupils may be constricted or dilated. Hypotension and tachycardia are common. Sometimes there can be cardiac arrhythmias, including ventricular fibrillation, conduction defects and cardiac arrest. Contraindications: Severe dystonic reactions have followed the use of haloperidol, particularly in children and adolescents. It should therefore be used with extreme care in children. Haloperidol may also cause severe neurotoxic reactions in patients with hyperthyroidism and in patients receiving lithium. Haloperidol is contraindicated in severe toxic central nervous system depression or comatose states from any cause and individuals who are hypersensitive to this drug or have Parkinson's disease. Also contraindicated in late pregnancy because of dystonic reaction in the neonate. Infants should not be nursed during drug treatment. Routes of entry: Oral: It is the main route of administration. Parenteral: Through intravenous and intramuscular injection. Absorption by route of exposure: Haloperidol is readily absorbed from the gastrointestinal tract. Owing to the first-pass effect of metabolism in the liver, plasma concentrations following oral administration are lower than those following intramuscular administration. There is wide intersubject variation in plasma concentration of haloperidol and its therapeutic effects. The decanoate ester of haloperidol is very slowly absorbed from the site of injection and is therefore suitable for depot injection. It is gradually released into the bloodstream where it is rapidly hydrolysed to haloperidol. Distribution by route of exposure: Haloperidol is very extensively bound to plasma proteins (90%). It is widely distributed in body and crosses the bloodbrain barrier. Biological half-life by route of exposure: The plasma half-life in therapeutic doses is reported to range from about 13 to nearly 40 hours (Reynolds, 1989), with a mean of 20 hours. Metabolism: Haloperidol is metabolized in the liver and the paths of metabolism include oxidative N-dealkylation. Elimination by route of exposure: This rate of total systemic clearance increases in children and decreases in aged patients. After metabolism, haloperidol is excreted in the urine, via the bile and in the feces, there is evidence of enterohepatic recycling by 40%. About 26% was excreted in the urine by the healthy subjects and 20% by the patients in the first 5 days; by the third day about 15% had been excreted in the feces. It takes 28 days to fully eliminate a single oral dose. Mode of action: Pharmacodynamics: Dopamine receptors currently are classified as D-1(stimulate adenylate cyclase) and D-2(inhibit adenylate cyclase). Neuroleptic drugs block both D-1 and D-2 receptors but the significance of the ratio remains unclear. The therapeutic dose of neuroleptic drug appears to correlate with its affinity for brain dopamine D-2 receptors. Neuroleptic drugs also block a number of other receptors including H1 and H2 histamine, alfa 1 and alfa 2 adrenergic, muscarinic and serotoninergic receptors. Toxicity: Human data: Three cases of sudden death after taking 20 to 140 mg daily for one to four days. Children: A 29-month-old girl and an 11 month old boy who divided 265 mg of haloperidol between them developed lethargy, hypothermia, hyperreflexia, neuromuscular rigidity, unsteady gait and intention tremors. Although disturbances such as galactorrhea, amenorrhea, gynaecomastia, and impotence have been reported, the clinical significance of elevated serum prolactin levels is unknown for most patients. There are no well controlled studies with haloperidol in pregnant women. There are reports, however, of cases of limb malformations observed following maternal use of haloperidol along with other drugs which have suspected teratogenic potential during the first trimester of pregnancy. Causal relationships were not established in these cases. Since such experience does not exclude the possibility of fetal damage due to haloperidol; this drug should be used during pregnancy or in women likely to become pregnant only if the benefit clearly justifies a potential risk to the fetus. Interactions: The use of alcohol with this drug should be avoided due to possible additive effects and hypotension. An encephalopathic syndrome (characterised by weakness, lethargy, fever, tremulousness and confusion, extrapyramidal symptoms, leucocytosis, elevated serum enzymes, BUN, and FBS) followed by irreversible brain damage has occurred in a few patients treated with lithium plus haloperidol. A casual relationship between these events and the concomitant administration of lithium and haloperidol has not been established; however, patients receiving such combination therapy should be monitored closely for early evidence of neurological toxicity and treatment discontinued promptly if such signs appear (Physician's Desk Reference, 1987). Other reported interactions involve the following drugs and adverse effects: Beta-blockers: Severe hypotension or pulmonary arrest. Methyldopa: Dementia, psychomotor retardation, memory impairment and inability to concentrate. Indomethacin: Severe drowsiness and confusion. Main adverse effects: In general, the symptoms of overdose would be an exaggeration of known pharmacological effects and adverse reactions. Anticholinergic side effects and sedation occur less often than with aliphatic phenothiazines, but extrapyramidal reactions are more common. Administration of antidopaminergic and anticholinergics may worsen or bring forward the onset of extrapyramidal effects. Idiosyncratic reaction producing severe drowsiness when used with indomethacin. ANIMAL/PLANT STUDIES: Carcinogenicity: Carcinogenicity studies using oral haloperidol were conducted in Wistar rats and in Albino Swiss mice. In the rat study, survival was less than optimal in all dose groups, reducing the number of rats at risk for developing tumors. However, although a relatively greater number of rats survived to the end of the study in high dose male and female groups, these animals did not have a greater incidence of tumors than control animals. Therefore, although not optimal, this study does suggest the absence of haloperidol related increase in the incidence of neoplasia in rats. In female mice there was a statistically significant increase in mammary gland neoplasia and total tumor incidence; there was a statistically significant increase in pituitary gland neoplasia. In male mice, no statistically significant differences in incidence of total tumors or specific tumor types were noted. Neuroleptic drugs elevate prolactin levels; the elevation persists during chronic administration. Teratogenicity: Rodents haloperidol by oral or parenteral routes showed an increase in incidence of resorption, reduced fertility, delayed delivery and pup mortality. No teratogenic effect has been reported in rats, rabbits or dogs at dosages within this range, but cleft palate has been observed in mice. Mutagenicity: No mutagenic potential of haloperidol was found in the Ames Salmonella microsomal activation assay. View MoreThe precise mechanism whereby the therapeutic effects of haloperidol are produced is not known, but the drug appears to depress the CNS at the subcortical level of the brain, midbrain, and brain stem reticular formation. Haloperidol seems to inhibit the ascending reticular activating system of the brain stem (possibly through the caudate nucleus), thereby interrupting the impulse between the diencephalon and the cortex. The drug may antagonize the actions of glutamic acid within the extrapyramidal system, and inhibitions of catecholamine receptors may also contribute to haloperidol's mechanism of action. Haloperidol may also inhibit the reuptake of various neurotransmitters in the midbrain, and appears to have a strong central antidopaminergic and weak central anticholinergic activity. The drug produces catalepsy and inhibits spontaneous motor activity and conditioned avoidance behaviours in animals. The exact mechanism of antiemetic action of haloperidol has also not been fully determined, but the drug has been shown to directly affect the chemoreceptor trigger zone (CTZ) through the blocking of dopamine receptors in the CTZ. Hepatotoxicity Liver test abnormalities have been reported to occur in 20% of patients on long term therapy with haloperidol, but elevations are uncommonly above 3 times the upper limit of normal. The aminotransferase abnormalities are usually mild, asymptomatic and transient, reversing even with continuation of medication. Instances of clinically apparent acute liver injury have been reported due to haloperidol, but they are uncommon. The onset of jaundice is within 2 to 6 weeks, and the pattern of serum enzyme elevations is typically cholestatic or mixed. Signs of hypersensitivity (fever, rash and eosinophilia) have been reported in some cases, but they are usually mild and self-limited; autoantibodies are rare. Likelihood score: B (likely cause of clinically apparent liver injury). Health Effects Tachycardia, hypotension, and hypertension; Extrapyramidal Symptoms (EPS) such as akathisia, or dystonia; impaired liver function and/or jaundice have been reported. Maculopapular and acneiform skin reactions and isolated cases of photosensitivity and loss of hair.Laryngospasm, bronchospasm, cataracts, retinopathy and visual disturbances; lactation, breast engorgement, mastalgia, menstrual irregularities, gynecomastia, impotence, increased libido, hyperglycemia, hypoglycemia and hyponatremia (RxList, A308). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Limited information indicates that maternal doses of haloperidol up to 10 mg daily produce low levels in milk and usually do not affect the breastfed infant. Very limited long-term follow-up data indicate no adverse developmental effects when haloperidol is used alone. However, use with other antipsychotic drugs occasionally might negatively affect the infant. One expert guideline recommends against using haloperidol during breastfeeding, but a safety scoring system finds haloperidol possible to use cautiously during breastfeeding. Monitor the infant for drowsiness and developmental milestones, especially if other antipsychotics are used concurrently. ◉ Effects in Breastfed Infants In one breast-fed infant, there were no sedative effects and the baby fed well during maternal intake of 5 mg orally twice daily. The mother took haloperidol during six weeks of breast feeding and at 6 and 12 months of age, the baby had achieved all milestones of growth and development. One infant was breastfed for 5 weeks beginning at 2 weeks of age during maternal haloperidol (dose not stated) and imipramine (150 mg daily) therapy. The infant showed normal development when tested once between 1 and 4 months and once between 12 and 18 months of age. In a small prospective study on the long-term effects of antipsychotics in breastfed infants, a decline in developmental scores was found at 12 to 18 months of age in 2 of the 4 the infants of mothers taking both chlorpromazine and haloperidol. The other 2 infants and all infants exposed to either drug alone developed normally. One woman with schizophrenia took haloperidol and trihexyphenidyl during 3 pregnancies and postpartum. Haloperidol doses were 7.5 to 10 mg daily in the first 2 pregnancies and 15 mg daily in the third. She breastfed (extent not stated) all 3 children for 6 to 8 months using the same doses. Development was age-appropriate in all children aged 16 months at 8 years of age at the time of assessment. Two women, one with bipolar disorder and the other with long-standing schizophrenia, were treated with haloperidol 5 mg daily during pregnancy and breastfeeding (extent not stated). One mother also received olanzapine 10 mg daily and the other received amisulpride 400 mg daily. Follow-up of the breastfed infants for 11 to 13 months found no adverse effects and normal development of the infants. A woman diagnosed with schizophrenia was taking risperidone 1.5 mg daily during late pregnancy and postpartum while nursing (extent not stated) her full-term infant. At 2 weeks postpartum, haloperidol 0.8 mg daily was added because of a recurrence of symptoms. At these dosages, no adverse effects were seen in the infant. However, because of recurring symptoms, the dosage of haloperidol was increased to 1.5 mg daily. Three days later, the infant had excessive sedation, poor feeding, and slowing in motor movements. Pediatric assessment found no medical reason for these effects. Breastfeeding was discontinued and the infant's symptoms resolved completely in 5 days. The infant's symptoms were probably caused by the drug combination. ◉ Effects on Lactation and Breastmilk Galactorrhea caused by hyperprolactinemia has been reported with haloperidol The hyperprolactinemia is caused by the drug's dopamine-blocking action in the tuberoinfundibular pathway. The maternal prolactin level in a mother with established lactation may not affect her ability to breastfeed. Drugs and Lactation Database (LactMed) ◈ What is haloperidol? Haloperidol is a medication that has been used to treat schizophrenia and other mental health conditions. It has also been used to treat a severe type of nausea and vomiting during pregnancy (hyperemesis gravidarum). More information on nausea and vomiting in pregnancy can be found here: https://mothertobaby.org/fact-sheets/nausea-vomiting-pregnancy-nvp/. A brand name for haloperidol is Haldol®.Sometimes when people find out they are pregnant, they think about changing how they take their medication, or stopping their medication altogether. However, it is important to talk with your healthcare providers before making any changes to how you take this medication. Your healthcare providers can talk with you about the benefits of treating your condition and the risks of untreated illness during pregnancy. ◈ I take haloperidol. Can it make it harder for me to get pregnant? Based on the studies reviewed, haloperidol may cause a higher level of prolactin (a hormone that helps the body make milk) in the blood than is usual. This is called hyperprolactinemia. Hyperprolactinemia may make it harder to get pregnant. ◈ Does taking haloperidol increase the chance for miscarriage? Miscarriage is common and can occur in any pregnancy for many different reasons. Studies have not been done in humans to see if haloperidol increases the chance for miscarriage. Based on animal studies, haloperidol is not expected to increase the chance for miscarriage. ◈ Does taking haloperidol increase the chance of birth defects? Every pregnancy starts out with a 3-5% chance of having a birth defect. This is called the background risk. Based on the studies reviewed, haloperidol is not expected to increase the chance for birth defects above the background risk. Most studies looking at the use of haloperidol during pregnancy did not find an increased chance for birth defects. There are two case reports of limb defects in infants after exposure to haloperidol and other medications during pregnancy. One study looking at 188 pregnancies exposed to haloperidol found no increased chance for birth defects. In this study, limb defects were reported in one infant. It is not known if haloperidol, other medications, or other factors caused the limb defects. ◈ Does taking haloperidol in pregnancy increase the chance of other pregnancy-related problems? Based on the studies reviewed, haloperidol is not expected to increase the chance of other pregnancy-related problems, such as preterm delivery (birth before week 37) or low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth). One study reported an increase in preterm delivery and lower birth weights when haloperidol was taken during pregnancy. However, the authors of the study reported they did not have information on some important factors that could be linked to low birth weight and/or preterm delivery. It is not known if haloperidol, other medications, or other factors increased the chance for these problems. ◈ I need to take haloperidol throughout my entire pregnancy. Will it cause withdrawal symptoms in my baby after birth? There have been reports of withdrawal symptoms in newborns exposed to haloperidol during pregnancy. Symptoms might include low muscle tone (floppy), restlessness, unusual sleep patterns, trouble eating, involuntary shaking movements (tremors), and dehydration. Not all babies exposed to haloperidol will have these symptoms. It is important that your healthcare providers know you are taking haloperidol so that if symptoms occur your baby can get the care that is best for them. ◈ Does taking haloperidol in pregnancy affect future behavior or learning for the child? Based on the studies reviewed, it is not known if haloperidol increases the chance for behavior or learning issues. ◈ Breastfeeding while taking haloperidol: Information on the use of haloperidol while breastfeeding is limited. Haloperidol passes into breastmilk. Most breastfed babies exposed to haloperidol have no reported symptoms.One breastfeeding infant reportedly had trouble with feeding, being too sleepy, and slow movements after exposure to haloperidol and risperidone through breastmilk. The baby’s symptoms went away after breastfeeding was stopped. The reported symptoms may be related to the combination of medications. If you suspect the baby has any symptoms (such as drowsiness), contact the child’s healthcare provider. Be sure to talk to your healthcare provider about all of your breastfeeding questions. ◈ If a male takes haloperidol, could it affect fertility (ability to get partner pregnant) or increase the chance of birth defects? Males who take haloperidol may develop hyperprolactinemia, which can cause problems with sexual desire or the ability to have an orgasm. Studies have not been done to see if haloperidol use by males could increase the chance of birth defects above the background risk. In general, exposures that fathers or sperm donors have are unlikely to increase the risks to a pregnancy. For more information, please see the MotherToBaby fact sheet Paternal Exposures at https://mothertobaby.org/fact-sheets/paternal-exposures-pregnancy/.A pregnancy registry for psychiatric medications, including this one, has been organized at the Massachusetts General Hospital. Contact the registry at https://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/. Exposure Routes Inhalation, Oral (60%) Symptoms LD50=165 mg/kg (rats, oral) Toxicity Data LD50: 128 mg/kg (Oral, Rat) LD50: 71 mg/kg (Oral, Mouse) LD50: 90 mg/kg (Oral, Dog) LD50: 165 mg/kg (Oral, Rat) Treatment Since there is no specific antidote, treatment is primarily supportive. A patent airway must be established by use of an oropharyngeal airway or endotracheal tube or, in prolonged cases of coma, by tracheostomy. Respiratory depression may be counteracted by artificial respiration and mechanical respirators. Hypotension and circulatory collapse may be counteracted by use of intravenous fluids, plasma, or concentrated albumin, and vasopressor agents such as metaraminol, phenylephrine and norepinephrine. Epinephrine should not be used. In case of severe extrapyramidal reactions, anti-Parkinson medication should be administered. ECG and vital signs should be monitored especially for signs of Q-T prolongation or dysrhythmias and monitoring should continue until the ECG is normal. Severe arrhythmias should be treated with appropriate anti-arrhythmic measures. (L1712) Non-Human Toxicity Values LD50 Rat oral 165 mg/kg LD50 Mouse ip 60 mg/kg Protein Binding Studies have found that free fraction of haloperidol in human plasma is 7.5-11.6%. This was found to be comparable among healthy adults, young adults, elderly patients with schizophrenia, and even in patients with liver cirrhosis. |
| 参考文献 |
[1]. Joy CB, et al. Haloperidol versus placebo for schizophrenia. Cochrane Database Syst Rev. 2006 Oct 18;(4):CD003082.
[2]. Giannini AJ, et al. Acute ketamine intoxication treated by haloperidol: a preliminary study. Am J Ther. 2000 Nov;7(6):389-91 |
| 其他信息 |
Haloperidol can cause developmental toxicity and female reproductive toxicity according to state or federal government labeling requirements.
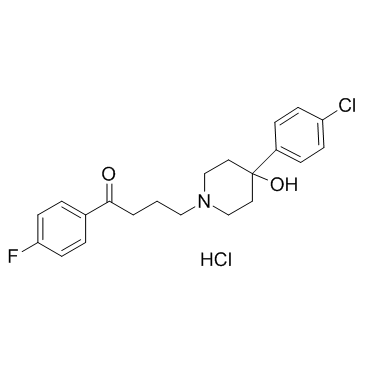
Haloperidol is a compound composed of a central piperidine structure with hydroxy and p-chlorophenyl substituents at position 4 and an N-linked p-fluorobutyrophenone moiety. It has a role as a serotonergic antagonist, a first generation antipsychotic, a dopaminergic antagonist, an antidyskinesia agent and an antiemetic. It is a hydroxypiperidine, an organofluorine compound, an aromatic ketone, a tertiary alcohol and a member of monochlorobenzenes. Haloperidol is a high potency first-generation (typical) antipsychotic and one of the most frequently used antipsychotic medications used worldwide. While haloperidol has demonstrated pharmacologic activity at a number of receptors in the brain, it exerts its antipsychotic effect through its strong antagonism of the dopamine receptor (mainly D2), particularly within the mesolimbic and mesocortical systems of the brain. Haloperidol is indicated for the treatment of the manifestations of several psychotic disorders including schizophrenia, acute psychosis, Tourette syndrome, and other severe behavioural states. It is also used off-label for the management of chorea associated with Huntington's disease and for the treatment of intractable hiccups as it is a potent antiemetic. Dopamine-antagonizing medications such as haloperidol are though to improve psychotic symptoms and states that are caused by an over-production of dopamine, such as schizophrenia, which is theorized to be caused by a hyperdopaminergic state within the limbic system of the brain. Use of the first-generation antipsychotics (including haloperidol) is considered highly effective for the management of the "positive" symptoms of schizophrenia including hallucinations, hearing voices, aggression/hostility, disorganized speech, and psychomotor agitation. However, this class of drugs is also limited by the development of movement disorders induced by dopamine-blockade such as drug-induced parkinsonism, akathisia, dystonia, tardive dyskinesia, as well as other side effects including sedation, weight gain, and prolactin changes. While there are limited high-quality studies comparing haloperidol to lower-potency first-generation antipsychotics such as [DB00477], [DB01624], [DB00623], and [DB01403], haloperidol typically demonstrates the least amount of side effects within this class, but demonstrates a stronger disposition for causing extrapyramidal symptoms (EPS). These other low‐potency antipsychotics are limited by their lower affinity for dopamine receptors, which requires a higher dose to effectively treat symptoms of schizophrenia. In addition, they block many receptors other than the primary target (dopamine receptors), such as cholinergic or histaminergic receptors, resulting in a higher incidence of side effects such as sedation, weight gain, and hypotension. Interestingly, in vivo pharmacogenetic studies have demonstrated that the metabolism of haloperidol may be modulated by genetically determined polymorphic CYP2D6 activity. However, these findings contradict the findings from studies in vitro with human liver microsomes and from drug interaction studies in vivo. Inter-ethnic and pharmacogenetic differences in haloperidol metabolism may possibly explain these observations. First-generation antipsychotic drugs have largely been replaced with second- and third-generation (atypical) antipsychotics such as [DB00734], [DB00334], [DB00363], [DB01224], [DB01238], and [DB00246]. However, haloperidol use remains widespread and is considered the benchmark for comparison in trials of the newer generation antipsychotics. The efficacy of haloperidol was first established in controlled trials in the 1960s. Haloperidol is a Typical Antipsychotic. View More
Haloperidol is a conventional antipsychotic agent used in the treatment of acute and chronic psychosis. Haloperidol therapy is commonly associated with minor serum aminotransferase elevations and in very rare instances has been linked to clinically apparent acute liver injury.
Drug Indication Haloperidol is indicated for a number of conditions including for the treatment of schizophrenia, for the manifestations of psychotic disorders, for the control of tics and vocal utterances of Tourette’s Disorder in children and adults, for treatment of severe behavior problems in children of combative, explosive hyperexcitability (which cannot be accounted for by immediate provocation). Haloperidol is also indicated in the short-term treatment of hyperactive children who show excessive motor activity with accompanying conduct disorders consisting of some or all of the following symptoms: impulsivity, difficulty sustaining attention, aggressivity, mood lability, and poor frustration tolerance. Haloperidol should be reserved for these two groups of children only after failure to respond to psychotherapy or medications other than antipsychotics. Therapeutic Uses Anti-Dyskinesia Agents; Antiemetics; Antipsychotic Agents, Butyrophenone; Dopamine Antagonists Haloperidol is indicated for the management of the manifestations of acute and chronic psychotic disorders including schizophrenia, manic states, and drug-induced psychoses, such as steroid psychosis. It may also be useful in the management of aggressive and agitated patients, including patients with organic mental syndrome or mental retardation. Haloperidol decanoate, a long-acting parenteral from, is intended for maintenance use in the management of patients requiring prolonged parenteral therapy, as in chronic schizophrenia. Haloperidol is effective in the treatment of children with severe behavior problems of apparently unprovoked, combative, explosive hyperexcitability. It is also effective in the short-term treatment of hyperactivity in children who show excessive motor activity with accompanying conduct disorders such as aggressiveness, impulsiveness, easy frustration, short attention span, and/or rapid mood fluctuations. In these two groups of children, haloperidol should be tried only in patients who fail to respond to psychotherapy or other non-neuroleptic medication. Haloperidol is used to control tics and vocalizations of Tourette's syndrome in children and adults. Drug Warnings Pregnancy risk category: C /RISK CANNOT BE RULED OUT. Adequate, well controlled human studies are lacking, and animal studies have shown risk to the fetus or are lacking as well. There is a chance of fetal harm if the drug is given during pregnancy; but the potential benefits may outweigh the potential risk./ Extrapyramidal reactions occur frequently with haloperidol, especially during the first few days of therapy. In most patients, these reactions consist of parkinsonian symptoms (e.g., marked drowsiness and lethargy, drooling or hypersalivation, fixed stare), which are mild to moderate in severity and are usually reversible following discontinuance of the drug. Other adverse neuromuscular reactions have been reported less frequently, but are often more severe, and include feelings of motor restlessness (i.e., akathisia), tardive dystonia, and dystonic reactions (e.g., hyperreflexia, opisthotonos, oculogyric crisis, torticollis, trismus). Possible drowsiness or dizziness; caution when driving, using machinery, or doing things requiring alertness. Possible dizziness or lightheadedness; caution when getting up suddenly from a lying or sitting position. Because of the possibility of transient hypotension and/or precipitation of angina, haloperidol should be used with caution in patients with severe cardiovascular disorders. If hypotension occurs, metaraminol, norepinephrine, or phenylephrine may be used; epinephrine should not be used since haloperidol causes a reversal of epinephrine's vasopressor effects and a further lowering of blood pressure. Pharmacodynamics Use of the first-generation antipsychotics (including haloperidol) is considered highly effective for the management of the "positive" symptoms of schizophrenia including hallucinations, hearing voices, aggression/hostility, disorganized speech, and psychomotor agitation. However, this class is limited by the development of movement disorders such as drug-induced parkinsonism, akathisia, dystonia, and tardive dyskinesia, and other side effects including sedation, weight gain, and prolactin changes. Compared to the lower-potency first-generation antipsychotics such as [DB00477], [DB01624], [DB00623], and [DB01403], haloperidol typically demonstrates the least amount of side effects within class, but demonstrates a stronger disposition for causing extrapyramidal symptoms (EPS). Low‐potency medications have a lower affinity for dopamine receptors so that a higher dose is required to effectively treat symptoms of schizophrenia. In addition, they block many receptors other than the primary target (dopamine receptors), such as cholinergic or histaminergic receptors, resulting in a higher incidence of side effects such as sedation, weight gain, and hypotension. The balance between the wanted drug effects on psychotic symptoms and unwanted side effects are largely at play within dopaminergic brain pathways affected by haloperidol. Cortical dopamine-D2-pathways play an important role in regulating these effects and include the nigrostriatal pathway, which is responsible for causing extrapyramidal symptoms (EPS), the mesolimbic and mesocortical pathways, which are responsible for the improvement in positive schizophrenic symptoms, and the tuberoinfundibular dopamine pathway, which is responsible for hyperprolactinemia. A syndrome consisting of potentially irreversible, involuntary, dyskinetic movements may develop in patients. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to rely upon prevalence estimates to predict, at the inception of antipsychotic treatment, which patients are likely to develop the syndrome. Cases of sudden death, QT-prolongation, and Torsades de Pointes have been reported in patients receiving haloperidol. Higher than recommended doses of any formulation and intravenous administration of haloperidol appear to be associated with a higher risk of QT-prolongation and Torsades de Pointes. Although cases have been reported even in the absence of predisposing factors, particular caution is advised in treating patients with other QT-prolonging conditions (including electrolyte imbalance [particularly hypokalemia and hypomagnesemia], drugs known to prolong QT, underlying cardiac abnormalities, hypothyroidism, and familial long QT-syndrome). A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with antipsychotic drugs. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status (including catatonic signs) and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmias). Additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis) and acute renal failure. Mechanism of Action While haloperidol has demonstrated pharmacologic activity at a number of receptors in the brain, it exerts its antipsychotic effect through its strong antagonism of the dopamine receptor (mainly D2), particularly within the mesolimbic and mesocortical systems of the brain. Schizophrenia is theorized to be caused by a hyperdopaminergic state within the limbic system of the brain. Dopamine-antagonizing medications such as haloperidol, therefore, are thought to improve psychotic symptoms by halting this over-production of dopamine. The optimal clinical efficacy of antipsychotics is associated with the blockade of approximately 60 % - 80 % of D2 receptors in the brain. While the exact mechanism is not entirely understood, haloperidol is known to inhibit the effects of dopamine and increase its turnover. Traditional antipsychotics, such as haloperidol, bind more tightly than dopamine itself to the dopamine D2 receptor, with dissociation constants that are lower than that for dopamine. It is believed that haloperidol competitively blocks post-synaptic dopamine (D2) receptors in the brain, eliminating dopamine neurotransmission and leading to the relief of delusions and hallucinations that are commonly associated with psychosis. It acts primarily on the D2-receptors and has some effect on 5-HT2 and α1-receptors, with negligible effects on dopamine D1-receptors. The drug also exerts some blockade of α-adrenergic receptors of the autonomic system. Antagonistic activity regulated through dopamine D2 receptors in the chemoreceptive trigger zone (CTZ) of the brain renders its antiemetic activity. Of the three D2-like receptors, only the D2 receptor is blocked by antipsychotic drugs in direct relation to their clinical antipsychotic abilities. Clinical brain-imaging findings show that haloperidol remains tightly bound to D2 dopamine receptors in humans undergoing 2 positron emission tomography (PET) scans with a 24h pause in between scans. A common adverse effect of this drug is the development of extrapyramidal symptoms (EPS), due to this tight binding of haloperidol to the dopamine D2 receptor. Due to the risk of unpleasant and sometimes lifelong extrapyramidal symptoms, newer antipsychotic medications than haloperidol have been discovered and formulated. Rapid dissociation of drugs from dopamine D2 receptors is a plausible explanation for the improved EPS profile of atypical antipsychotics such as [DB00734]. This is also consistent with the theory of a lower affinity for D2 receptors for these drugs. As mentioned above, haloperidol binds tightly to the dopamine receptor, potentiating the risk of extrapyramidal symptoms, and therefore should only been used when necessary. Haloperidol has less prominent autonomic effects than do other antipsychotic drugs. It has little anticholinergic activity ... it blocks activation of alpha receptors by sympathomimetic amines but is much less potent than chlorpromazine in this action. Although the complex mechanism of the therapeutic effect is not clearly established, haloperidol is known to produce a selective effect on the central nervous system (CNS) by competitive blockade of postsynaptic dopamine (D2) receptors in the mesolimbic dopaminergic system and an increased turnover of brain dopamine to produce its tranquilizing effects. With subchronic therapy, depolarization blockade, or diminished firing rate of the dopamine neuron (decreased release) along with D2 postsynaptic blockade results in the antipsychotic action. |
| 分子式 |
C21H23NO2FCL.HCL
|
|---|---|
| 分子量 |
412.32516
|
| 精确质量 |
411.117
|
| CAS号 |
1511-16-6
|
| 相关CAS号 |
Haloperidol;52-86-8;Haloperidol-d4;1189986-59-1;Haloperidol-d4-1;136765-35-0;Haloperidol lactate;53515-91-6
|
| PubChem CID |
11495267
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| LogP |
5.165
|
| tPSA |
40.54
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
27
|
| 分子复杂度/Complexity |
451
|
| 定义原子立体中心数目 |
0
|
| SMILES |
ClC(C=C1)=CC=C1C2(O)CCN(CCCC(C3=CC=C(F)C=C3)=O)CC2.Cl
|
| InChi Key |
JMRYYMBDXNZQMH-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C21H23ClFNO2.ClH/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16;/h3-10,26H,1-2,11-15H2;1H
|
| 化学名 |
4-[4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl]-1-(4-fluorophenyl)butan-1-one;hydrochloride
|
| 别名 |
haloperidol hydrochloride; 1511-16-6; Haloperidol chloride; Haloperidol (hydrochloride); Haloperidol chlorohydrate; Haloperidol hydrochloride [MI]; UNII-UM06W2ADRY; UM06W2ADRY;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4252 mL | 12.1262 mL | 24.2524 mL | |
| 5 mM | 0.4850 mL | 2.4252 mL | 4.8505 mL | |
| 10 mM | 0.2425 mL | 1.2126 mL | 2.4252 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03021486 | Active Recruiting |
Other: Chlorpromazine Drug: Haloperidol |
Delirium Advanced Malignant Neoplasm |
M.D. Anderson Cancer Center | June 5, 2017 | Phase 2 Phase 3 |
| NCT01949662 | Active Recruiting |
Drug: Placebo Drug: Haloperidol decanoate |
Advanced Cancers | M.D. Anderson Cancer Center | January 2014 | Phase 2 |
| NCT03392376 | Active Recruiting |
Drug: Haloperidol Injection Other: Saline (0,9%) |
Delirium | Zealand University Hospital | June 13, 2018 | Phase 4 |
| NCT04750395 | Recruiting | Drug: Haloperidol Solution Drug: Olanzapine Tablets |
Delirium Terminal Illness |
HCA Hospice Care | September 1, 2021 | Phase 2 |
| NCT03743649 | Recruiting | Drug: Haloperidol Drug: Lorazepam |
Delirium Metastatic Malignant Neoplasm |
M.D. Anderson Cancer Center | July 17, 2019 | Phase 2 Phase 3 |