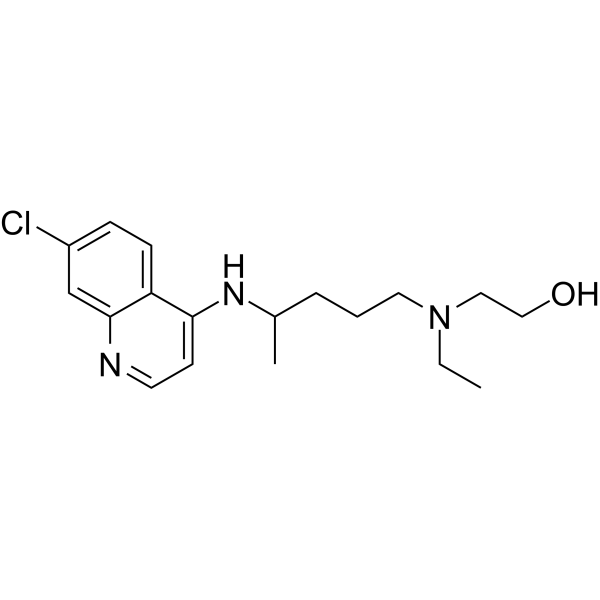
羟氯喹是一种合成抗疟药,还可抑制 Toll 样受体 7/9 (TLR7/9) 信号传导。羟氯喹可有效抑制 SARS-CoV-2 感染。
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
Binimetinib and Hydroxychloroquine in Treating Patients with KRAS Mutant Metastatic Pancreatic Cancer
CTID: NCT04132505
Phase: Phase 1 Status: Active, not recruiting
Date: 2024-12-02
A Study of Nivolumab and Hydroxychloroquine or Nivolumab/Ipilimumab and Hydroxychloroquine in Advanced Melanoma
CTID: NCT04464759
Phase: Phase 1/Phase 2 Status: Recruiting
Date: 2024-11-21
Therapeutic Effect of Hydroxychloroquine on Immunoglobulin A (IgA) Nephropathy Course QUIgAN Study
CTID: NCT06350630
Phase: Phase 2 Status: Not yet recruiting
Date: 2024-11-19
Ulixertinib (BVD-523) and Hydroxychloroquine in Patients W Advanced MAPK-Mutated Gastrointestinal Adenocarcinomas
CTID: NCT04145297
Phase: Phase 1 Status: Completed
Date: 2024-11-18
New Clinical End-points in Patients With Primary Sjögren's Syndrome
CTID: NCT05113004
Phase: Phase 2 Status: Recruiting
Date: 2024-11-01
View More
Finding Treatments for COVID-19: A Trial of Antiviral Pharmacodynamics in Early Symptomatic COVID-19 (PLATCOV)
CTID: NCT05041907
Phase: Phase 2 Status: Recruiting
Date: 2024-10-28
Preemptive Therapy for SARS-Coronavirus-2 (COVID-19 PEP Canada)
CTID: NCT04421664
Phase: Phase 3 Status: Terminated
Date: 2024-10-15
Study of Paclitaxel Protein Bound + Gemcitabine + Cisplatin + Hydrochloroquine as Treatment in Untreated Pancreas Cancer
CTID: NCT04669197
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-10-15
The Effects of Hydroxychloroquine in Patients with Inflammatory Cardiomyopathy
CTID: NCT05961202
Phase: Phase 2/Phase 3 Status: Recruiting
Date: 2024-10-09
PaTcH Study: A Phase 2 Study of Trametinib and Hydroxychloroquine in Patients With Metastatic Refractory Pancreatic Cancer
CTID: NCT05518110
Phase: Phase 2 Status: Recruiting
Date: 2024-09-26
Hydroxychloroquine Post Exposure Prophylaxis for Coronavirus Disease (COVID-19)
CTID: NCT04318444
Phase: Phase 2/Phase 3 Status: Terminated
Date: 2024-09-25
A Study of Hydroxychloroquine, Vitamin C, Vitamin D, and Zinc for the Prevention of COVID-19 Infection
CTID: NCT04335084
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-09-24
Akt Inhibitor MK2206 and Hydroxychloroquine in Treating Patients With Advanced Solid Tumors, Melanoma, Prostate or Kidney Cancer
CTID: NCT01480154
Phase: Phase 1 Status: Active, not recruiting
Date: 2024-09-19
Treatment of Rheumatoid Arthritis With DMARDs: Predictors of Response
CTID: NCT03414502
Phase: Phase 3 Status: Recruiting
Date: 2024-09-19
Trial of Treatments for COVID-19 in Hospitalized Adults
CTID: NCT04315948
Phase: Phase 3 Status: Completed
Date: 2024-09-19
Testing Dabrafenib and Trametinib With or Without Hydroxychloroquine in Stage IIIC or IV BRAF V600E/K Melanoma
CTID: NCT04527549
Phase: Phase 2 Status: Terminated
Date: 2024-08-22
Hydroxychloroquine and Cognitive Function After Surgery
CTID: NCT03025087
Phase: Phase 4 Status: Terminated
Date: 2024-08-20
Gemcitabine, Docetaxel, and Hydroxychloroquine in Treating Participants With Recurrent or Refractory Osteosarcoma
CTID: NCT03598595
Phase: Phase 1/Phase 2 Status: Active, not recruiting
Date: 2024-08-19
Sorafenib Induced Autophagy Using Hydroxychloroquine in Hepatocellular Cancer
CTID: NCT03037437
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-08-13
Trial of Ulixertinib in Combination With Hydroxychloroquine in Patients With Advanced Gastrointestinal (GI) Malignancies
CTID: NCT05221320
Phase: Phase 2 Status: Terminated
Date: 2024-07-22
a Retrospective Study on the Systemic Treatment of LPP and FFA
CTID: NCT06512766
Phase: Status: Completed
Date: 2024-07-22
The Effectiveness of Hydroxychloroquine Versus Methotrexate in the Treatment of Lichen Planopilaris in Routine Clinical Care: a Patient Preference Trial
CTID: NCT06512753
Phase: Status: Recruiting
Date: 2024-07-22
Hydroxychloroquine in ANCA Vasculitis Evaluation
CTID: NCT04316494
Phase: Phase 4 Status: Active, not recruiting
Date: 2024-07-19
Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community- Acquired Pneumonia
CTID: NCT02735707
Phase: Phase 3 Status: Recruiting
Date: 2024-07-12
IL-7 and IL-7R Expression in RA Patients With Active vs. Inactive Disease Treated With DMARD or CIMZIA
CTID: NCT02451748
Phase: Phase 4 Status: Completed
Date: 2024-07-10
Hydroxychloroquine in Children's Interstitial Lung Diseases With Genetic Causes
CTID: NCT04532346
PhaseEarly Phase 1 Status: Not yet recruiting
Date: 2024-07-09
Management of Cardiovascular Disease in Kidney Disease (MaCK) Study
CTID: NCT03636152
Phase: Phase 2 Status: Completed
Date: 2024-07-03
The Efficacy and Safety of HCQ Plus Pred in ANA Positive ITP
CTID: NCT06479304
Phase: N/A Status: Recruiting
Date: 2024-06-28
The Efficacy and Safety of HCQ Plus DEX in ANA Positive ITP
CTID: NCT06479317
Phase: N/A Status: Recruiting
Date: 2024-06-28
The Efficacy and Safety of HCQ Plus TPO-RA in ANA Positive ITP
CTID: NCT06479291
Phase: N/A Status: Recruiting
Date: 2024-06-28
Effects of Stopping Hydroxychloroquine in Elderly Lupus Disease
CTID: NCT05799378
Phase: Phase 3 Status: Recruiting
Date: 2024-06-28
Efficacy and Safety of Hydroxychloroquine in the Treatment of Sudden Sensorineural Hearing Loss
CTID: NCT06467526
Phase: Phase 2 Status: Not yet recruiting
Date: 2024-06-21
Efficacy and Safety of Baricitinib in Sjogren's Syndrome
CTID: NCT05016297
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-05-29
Hydroxychloroquine in Type 2 Diabetes During Pregnancy
CTID: NCT06319560
Phase: N/A Status: Recruiting
Date: 2024-05-29
Pretreatment With HCQ Before Radiotherapy and Chemotherapy in Advanced NPC Patients
CTID: NCT06389201
Phase: N/A Status: Not yet recruiting
Date: 2024-04-29
Trametinib and Hydroxychloroquine in Treating Patients With Pancreatic Cancer
CTID: NCT03825289
Phase: Phase 1 Status: Recruiting
Date: 2024-04-19
Hydroxychloroquine, Palbociclib, and Letrozole Before Surgery in Treating Patients With Estrogen Receptor Positive, HER2 Negative Breast Cancer
CTID: NCT03774472
Phase: Phase 1/Phase 2 Status: Completed
Date: 2024-04-19
Hydroxychloroquine in Individuals At-risk for Type 1 Diabetes Mellitus
CTID: NCT03428945
Phase: Phase 2 Status: Terminated
Date: 2024-04-09
A Trial of Dabrafenib, Trametinib and Hydroxychloroquine for Patients With Recurrent LGG or HGG With a BRAF Aberration
CTID: NCT04201457
Phase: Phase 1/Phase 2 Status: Recruiting
Date: 2024-04-05
HCQ+ADC vs ADC in the Treatment of Advanced Breast Cancer
CTID: NCT06328387
Phase: Phase 1/Phase 2 Status: Recruiting
Date: 2024-03-25
ABemacicliB or Abemaciclib and HydroxYchloroquine to Target Minimal Residual Disease in Breast Cancer
CTID: NCT04523857
Phase: Phase 2 Status: Recruiting
Date: 2024-03-12
Avelumab or Hydroxychloroquine With or Without Palbociclib to Eliminate Dormant Breast Cancer
CTID: NCT04841148
Phase: Phase 2 Status: Recruiting
Date: 2024-03-12
CLEVER Pilot Trial: A Phase II Pilot Trial of HydroxyChLoroquine, EVErolimus or the Combination for Prevention of Recurrent Breast Cancer
CTID: NCT03032406
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-03-12
Evaluation of Hydroxychloroquine to Prevent CIPN
CTID: NCT05689359
Phase: Phase 2 Status: Withdrawn
Date: 2024-03-08
Hydroxychloroquine as a Steroid-sparing Agent in Extrapulmonary Sarcoidosis
CTID: NCT05841758
Phase: Phase 4 Status: Not yet recruiting
Date: 2024-03-06
Administration of Hydroxychloroquine (Plaquenil) to African Americans and Hispanics for the Treatment of Mild to Severe Ulcerative Colitis
CTID: NCT05119140
Phase: Phase 1/Phase 2 Status: Active, not recruiting
Date: 2024-03-04
A Study of Quintuple Therapy to Treat COVID-19 Infection
CTID: NCT04334512
Phase: Phase 2 Status: Completed
Date: 2024-02-29
Study of Combination Therapy With the MEK Inhibitor, Cobimetinib, Immune Checkpoint Blockade, Atezolizumab, and the AUTOphagy Inhibitor, Hydroxychloroquine in KRAS-mutated Advanced Malignancies
CTID: NCT04214418
Phase: Phase 1/Phase 2 Status: Active, not recruiting
Date: 2024-02-28
Targeting Ischemia-Induced Autophagy Dependence in Hepatocellular Carcinoma
CTID: NCT05842174
Phase: Phase 1/Phase 2 Status: Not yet recruiting
Date: 2024-02-13
Study of Hydroxychloroquine With FOLFIRI and Bevacizumab in DTP-high Metastatic Colorectal Cancer
CTID: NCT05843188
Phase: Phase 2 Status: Recruiting
Date: 2024-02-09
Immune-Mediated Pathophysiology And Clinical Triage Program
CTID: NCT04354649
Phase: Phase 2 Status: Recruiting
Date: 2024-01-31
Multi-omics Studies on the Efficacy of Telitacicept in Chinese SLE Patients
CTID: NCT05666336
Phase: Phase 4 Status: Recruiting
Date: 2024-01-17
Hydroxychloroquine in Mild Graves' Orbitopathy
CTID: NCT05126147
Phase: Phase 4 Status: Recruiting
Date: 2024-01-17
Paricalcitol and Hydroxychloroquine in Combination With Gemcitabine and Nab-Paclitaxel for Advanced Pancreatic Cancer
CTID: NCT04524702
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-01-11
Vorinostat Plus Hydroxychloroquine Versus Regorafenib in Colorectal Cancer
CTID: NCT02316340
Phase: Phase 2 Status: Completed
Date: 2024-01-05
Randomised Evaluation of COVID-19 Therapy
CTID: NCT04381936
Phase: Phase 3 Status: Recruiting
Date: 2024-01-05
Study of Anti-Malarials in Incomplete Lupus Erythematosus
CTID: NCT03030118
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-01-03
Metabolic Effects of Hydroxychloroquine
CTID: NCT02026232
Phase: N/A Status: Terminated
Date: 2023-12-28
Strategy to Prevent the Onset of Clinically-Apparent Rheumatoid Arthritis
CTID: NCT02603146
Phase: Phase 2 Status: Terminated
Date: 2023-12-20
Hydroxychloroquine in Prevention of Preeclampsia
CTID: NCT04755322
Phase: N/A Status: Completed
Date: 2023-12-08
Hydroxychloroquine and Unexplained Recurrent Miscarriage
CTID: NCT04228263
Phase: N/A Status: Completed
Date: 2023-12-07
The Efficacy of Aspirin Combined With Hydroxychloroquine Treatment in High Risk Pregnancies for Preeclampsia
CTID: NCT05287321
Phase: Phase 3 Status: Recruiting
Date: 2023-11-28
Safety and Effect on Pain and Function According to RAPID-3 of IHL-675A in Patients With Rheumatoid Arthritis
CTID: NCT05942911
Phase: Phase 2 Status: Recruiting
Date: 2023-11-27
Phase I/II Study of Hydroxychloroquine With Itraconazole With Biochemically Recurrent Prostate Cancer
CTID: NCT03513211
Phase: Phase 1/Phase 2 Status: Completed
Date: 2023-11-22
The Clinical Features and Pregnancy Outcomes of CTD Patients
CTID: NCT04918524
Phase: Status: Recruiting
Date: 2023-11-18
The Clinical Efficacy and Safety of Iguratimod in RA and Early RA Patients for 6 Months Treatment
CTID: NCT03855007
Phase: Phase 4 Status: Completed
Date: 2023-11-18
To Determine the Safety of Regorafenib, Hydroxychloroquine, and Entinostat Metastatic Colorectal Cancer
CTID: NCT03215264
Phase: Phase 1 Status: Completed
Date: 2023-11-09
Hydroxychloroquine for Prevention of Recurrent Miscarriage.
CTID: NCT03165136
Phase: Phase 3 Status: Active, not recruiting
Date: 2023-10-02
Non-comparative Trial of the Combination of HCQ and AZI in the Treatment of ICU Patients
CTID: NCT04458948
Phase: Phase 2 Status: Terminated
Date: 2023-09-13
Hydroxychloroquine May be Beneficial for Preeclampsia
CTID: NCT06020378
Phase: Status: Not yet recruiting
Date: 2023-08-31
Combination of Trametinib (MEK Inhibitor) and Hydroxychloroquine (HCQ) (Autophagy Inhibitor) in Patients With KRAS Mutation Refractory Bile Tract Carcinoma (BTC).
CTID: NCT04566133
Phase: Phase 2 Status: Terminated
Date: 2023-08-21
Docetaxel and Hydroxychloroquine in Treating Patients With Metastatic Prostate Cancer
CTID: NCT00786682
Phase: Phase 2 Status: Terminated
Date: 2023-08-14
Ixabepilone and Hydroxychloroquine in Treating Patients With Metastatic Breast Cancer
CTID: NCT00765765
Phase: Phase 1/Phase 2 Status: Terminated
Date: 2023-08-09
Sunitinib Malate and Hydroxychloroquine in Treating Patients With Advanced Solid Tumors That Have Not Responded to Chemotherapy
CTID: NCT00813423
Phase: Phase 1 Status: Completed
Date: 2023-08-02
A Study of Nipocalimab With Co-administration of Etanercept or Hydroxychloroquine in Healthy Participants
CTID: NCT04973566
Phase: Phase 1 Status: Completed
Date: 2023-07-03
Efficacy of VHM After Treatment Interruption in Subjects Initiating ART During Acute HIV Infection
CTID: NCT02475915
Phase: Phase 1/Phase 2 Status: Completed
Date: 2023-06-22
To Evaluate Maximally Tolerated Dose (MTD), Safety and Efficacy of CPI-613® (Devimistat) Plus Hydroxychloroquine in Patients With Relapsed or Refractory Clear Cell Sarcoma of Soft Tissue
CTID: NCT04593758
Phase: Phase 1/Phase 2 Status: Completed
Date: 2023-05-24
Treatment of Muscle Cramps in Patients With Liver Cirrhosis
CTID: NCT01495403
PhaseEarly Phase 1 Status: Completed
Date: 2023-05-03
Pre-Exposure Prophylaxis With Hydroxychloroquine for High-Risk Healthcare Workers During the COVID-19 Pandemic
CTID: NCT04331834
Phase: Phase 3 Status: Completed
Date: 2023-05-01
CPI-613 (Devimistat) in Combination With Hydroxychloroquine and 5-fluorouracil or Gemcitabine in Treating Patients With Advanced Chemorefractory Solid Tumors
CTID: NCT05733000
Phase: Phase 2 Status: Recruiting
Date: 2023-03-10
Using Hydroxychloroquine to Treat Nonalcoholic Steatohepatitis
CTID: NCT05733897
Phase: Status: Recruiting
Date: 2023-03-02
Chemoprophylaxis of SARS-CoV-2 Infection (COVID-19) in Exposed Healthcare Workers
CTID: NCT04328285
Phase: Phase 3 Status: Terminated
Date: 2023-03-01
High-dose Hydroxychloroquine for the Treatment of Ambulatory Patients With Mild COVID-19
CTID: NCT04351620
Phase: Phase 1 Status: Completed
Date: 2023-02-23
Hydroxychloroquine in Colchicine-Resistant Glucocorticoid-Dependent Idiopathic Recurrent Pericarditis
CTID: NCT05737680
Phase: Phase 3 Status: Not yet recruiting
Date: 2023-02-22
Hydroxychloroquine and Phlebotomy for Treating Porphyria Cutanea Tarda
CTID: NCT01573754
Phase: Phase 2 Status: Completed
Date: 2023-02-17
University of Utah COVID-19 Hydrochloroquine Trial
CTID: NCT04342169
Phase: Phase 2 Status: Completed
Date: 2023-02-15
Hydroxychloroquine + Vorinostat in Advanced Solid Tumors
CTID: NCT01023737
Phase: Phase 1 Status: Completed
Date: 2023-02-13
Tacrolimus for Thrombocytopenia in SS
CTID: NCT05678335
Phase: Phase 2/Phase 3 Status: Unknown status
Date: 2023-01-10
Hydroxychloroquine, Abemaciclib and Endocrine Therapy in Hormone Receptor Positive (HR+)/Her 2 Negative Breast Cancer
CTID: NCT04316169
Phase: Phase 1 Status: Withdrawn
Date: 2022-12-23
The Clinical Features and Pregnancy Outcomes of RA Patients
CTID: NCT05651373
Phase: Status: Recruiting
Date: 2022-12-15
COVID-19 PrEP HCW HCQ Study
CTID: NCT04354870
Phase: Phase 2 Status: Completed
Date: 2022-12-14
Telitacicept Followed With Rituximab Therapy on APS Secondary to SLE
CTID: NCT05644210
Phase: Status: Recruiting
Date: 2022-12-09
The Clinical Efficacy of Immunomodulators in RA Patients
CTID: NCT05626348
Phase: Phase 4 Status: Recruiting
Date: 2022-11-29
Modulation of Autophagy in Patients With Advanced/Recurrent Non-small Cell Lung Cancer - Phase II
CTID: NCT01649947
Phase: Phase 2 Status: Completed
Date: 2022-11-21
Treatments Against RA and Effect on FDG-PET/CT
CTID: NCT02374021
Phase: Phase 4 Status: Completed
Date: 2022-10-26
Hydroxychloroquine Versus Pioglitazone in Combination Treatment for Type 2 Diabetes Mellitus
CTID: NCT02303405
Phase: Phase 2 Status: Terminated
Date: 2022-10-20
Prediction of Relapse Risk in Stable Systemic Lupus Erythematosus
CTID: NCT02842814
Phase: N/A Status: Completed
Date: 2022-10-03
Hydroxychloroquine in Patients With Stage III or Stage IV Melanoma That Can Be Removed by Surgery
CTID: NCT00962845
PhaseEarly Phase 1 Status: Completed
Date: 2022-09-07
Efficacy and Safety of Mucoadhesive Sustained Release, Mucodentol, in Comparison With Hydroxychloroquine to Prevent COVID-19
CTID: NCT04466280
Phase: N/A Status: Withdrawn
Date: 2022-08-03
Clinical Trial Evaluating Methotrexate or Leflunomide + Targeted Therapy Versus Methotrexate or Leflunomide + Sulfasalazine + Hydroxychloroquine in Patients With Rheumatoid Arthritis and Insufficient Response to Methotrexate or Leflunomide
CTID: NCT02714634
Phase: Phase 4 Status: Recruiting
Date: 2022-07-12
Efficacy of Hydroxychloroquine, Telmisartan and Azithromycin on the Survival of Hospitalized Elderly Patients With COVID-19
CTID: NCT04359953
Phase: Phase 3 Status: Terminated
Date: 2022-07-11
Hydroxychloroquine in Treating Patients With Rising PSA Levels After Local Therapy for Prostate Cancer
CTID: NCT00726596
Phase: Phase 2 Status: Completed
Date: 2022-06-16
A Triple Combination Antiviral Coronavirus Therapy (TriACT) for COVID-19
CTID: NCT04605588
Phase: Phase 2 Status: Terminated
Date: 2022-05-05
Effect of Sarilumab on Patient-reported Outcomes in Patients With Active Rheumatoid Arthritis
CTID: NCT03449758
Phase: Phase 4 Status: Completed
Date: 2022-04-28
Treating COVID-19 With Hydroxychloroquine (TEACH)
CTID: NCT04369742
Phase: Phase 2 Status: Terminated
Date: 2022-04-22
Hydroxychloroquine vs. Azithromycin for Hospitalized Patients With Suspected or Confirmed COVID-19
CTID: NCT04329832
Phase: Phase 2 Status: Completed
Date: 2022-04-20
HC
Leflunomide and Hydroxychloroquine combination therapy
CTID: null
Phase: Phase 2 Status: Ongoing
Date: 2021-06-10
Hydroxychloroquine in isolated cutaneous mastocytosis patients or indolent systemic mastocytosis with associated skin involvement patients: proof of concept study
CTID: null
Phase: Phase 2 Status: Trial now transitioned
Date: 2020-11-09
An international randomised trial of additional treatments for COVID-19 in hospitalised patients who are all receiving the local standard of care
CTID: null
Phase: Phase 3 Status: Prematurely Ended
Date: 2020-06-22
Pragmatic study 'CORIVER': Ivermectin as antiviral treatment for patients infected by SARS-COV2 (COVID-19)
CTID: null
Phase: Phase 3 Status: Ongoing
Date: 2020-06-02
Effectiveness of the combined treatment with hydroxycloroquine and azithromycin vs lopinavir/ritonavir + hydroxycloroquine in hospitalized patients with confirmed COVID-19 infection
CTID: null
Phase: Phase 4 Status: Prematurely Ended
Date: 2020-05-29
Prevention of COVID19 infection with hydroxychloroquine in institutionalized older people and nursing home staff. An open, randomized controlled stepped-wedge clinical trial by clusters.
CTID: null
Phase: Phase 3 Status: Ongoing
Date: 2020-05-22
Controlled and randomised trial to assess the safety and efficacy of hydroxychloroquine chemoprophylaxis in SARS CoV2 infection in hospital healthcare personnel (Sanitarios sin COVID-19 -SANsinCOVID-19).
CTID: null
Phase: Phase 4 Status: Prematurely Ended
Date: 2020-05-15
TOFAcitinib plus Hydroxycloroquine vs Hydroxycloroquine in patients with early onset SARS-CoV2 (COVID-19) interstitial pneumonia: a multicenter randomized controlled open label trial
CTID: null
Phase: Phase 2 Status: Completed
Date: 2020-05-15
Adaptive Randomized trial for therapy of COrona virus disease 2019 at home with oral antivirals
CTID: null
Phase: Phase 3 Status: Prematurely Ended
Date: 2020-05-14
Randomized, Controlled, Double-blind Clinical Trial Comparing the Efficacy
CTID: null
Phase: Phase 4 Status: Prematurely Ended
Date: 2020-05-13
PRECOV: a randomized controlled clinical trial on the effects of hydroxychloroquine in the prevention of COVID-19 in healthcare workers at risk
CTID: null
Phase: Phase 3 Status: Ongoing
Date: 2020-05-06
A multicentre, prospective, randomised trial comparing standard of care (SOC) alone, SOC plus hydroxychloroquine monotherapy or SOC plus a combination of hydroxychloroquine and azithromycin in the treatment of non-critical, SARS-CoV-2 PCR-positive population not requiring immediate resuscitation or ventilation but who have evidence of clinical decline.
CTID: null
Phase: Phase 4 Status: Prematurely Ended
Date: 2020-05-05
EARLY TREATMENT OF PNEUMONIA COVID-19 WITH GLUCOCORTICOIDS.
CTID: null
Phase: Phase 3 Status: Ongoing
Date: 2020-04-30
A Randomised Controlled Trial of Early Intervention in Patients HospItalised with COVID-19: Favipiravir verses HydroxycholorquiNe & Azithromycin & Zinc vErsEs Standard CaRe
CTID: null
Phase: Phase 3 Status: GB - no longer in EU/EEA
Date: 2020-04-29
CLINICAL TRIAL OF THE USE OF ANAKINRA (ANTI IL-1) IN CYTOKINE STORM SYNDROME (CSS) SECONDARY TO COVID-19
CTID: null
Phase: Phase 2, Phase 3 Status: Ongoing
Date: 2020-04-28
PROTECT: A randomized study with Hydroxychloroquine versus observational support for prevention or early phase treatment of Coronavirus disease (COVID-19).
CTID: null
Phase: Phase 2 Status: Prematurely Ended
Date: 2020-04-28
Cumulative adaptive, multiarm, multistage and multicentre randomized clinical trial with immunotherapy for Moderate COVID-19
CTID: null
Phase: Phase 2, Phase 3 Status: Prematurely Ended
Date: 2020-04-27
PRE-EXPOSURE PROPHYLAXIS WITH HYDROXYCHLOROQUINE IN SANITARIES HIGHLY EXPOSED TO COVID-19. (COVIDNA)
CTID: null
Phase: Phase 3 Status: Ongoing
Date: 2020-04-23
COVID-19 Prophylaxis with hydroxychloroquine, Vitamin D, and Zinc supplementation in Danish nursing home residents – a randomized controlled trial
CTID: null
Phase: Phase 3 Status: Prematurely Ended
Date: 2020-04-23
Phase I/II clinical trial to evaluate the safety and efficacy of Allogenic Adipose Tissue-Derived Mesenchymal Stem Cells Expanded in patients with severe COVID-19 pneumonia
CTID: null
Phase: Phase 1, Phase 2 Status: Ongoing
Date: 2020-04-20
Pilot, double-blind clinical trial to evaluate the efficacy and safety of pre-exposure use of hydroxychloroquine versus placebo in the prevention of SARS-CoV-2 (COVID-19) infection in healthcare personnel.
CTID: null
Phase: Phase 3 Status: Prematurely Ended
Date: 2020-04-19
Prophylaxis of COVID-19 infection with hydroxychloroquine in healthcare
CTID: null
Phase: Phase 3 Status: Prematurely Ended
Date: 2020-04-18
The Danish Pre-HCQ Dialysis Study:
CTID: null
Phase: Phase 3 Status: Prematurely Ended
Date: 2020-04-15
Efficacy and safety of novel treatment options for adults with COVID-19 pneumonia. A double-blinded, randomized, multi-stage, 6-armed placebo-controlled trial in the framework of an adaptive trial platform
CTID: null
Phase: Phase 3 Status: Prematurely Ended
Date: 2020-04-14
ChemoPROphyLaxIs For covId-19 infeCtious disease (the PROLIFIC trial)
CTID: null
Phase: Phase 3 Status: GB - no longer in EU/EEA
Date: 2020-04-14
An open label single center randomized controlled trial to evaluate the effect of hydroxychloroquine on viral shedding in mild COVID-19
CTID: null
Phase: Phase 2 Status: Prematurely Ended
Date: 2020-04-12
Efficacy and safety of sarilumab in the early treament of hospitalized patients with mild-moderate neumonia and COVID19 infection versus standard of care
CTID: null
Phase: Phase 3 Status: Prematurely Ended
Date: 2020-04-11
Efficacy of Hydroxychloroquine, Telmisartan and Azithromycin on Survival in Elderly Hospitalized Patients with VIDOC-19 : A Randomized, Multi-Centre, Adaptive, Blinded Study
CTID: null
Phase: Phase 3 Status: Prematurely Ended
Date: 2020-04-11
Pragmatic clinical trial to evaluate the efficacy of hydroxychloroquine in the treatment of COVID-19 infection in two cohorts: patients with oncohaematological disease and SARS-CoV-2 positive without radiological alteration and sars-cov-2 positive professionals without radiological alteration
CTID: null
Phase: Phase 2 Status: Prematurely Ended
Date: 2020-04-11
Prospective, phase II, randomized, open-label, parallel group study to evaluate the efficacy of hydroxychloroquine together with baricitinib, imatinib or early lopinavir / ritonavir in patients with SARS Cov2 pneumonia (COVID-19 HUF)
CTID: null
Phase: Phase 2 Status: Completed
Date: 2020-04-11
COVID-19+
CTID: null
Phase: Phase 3 Status: Prematurely Ended
Date: 2020-04-10
Hydroxychloroquine sulfate early administration in symptomatic out of hospital COVID-19 positive patients. Hydro-Stop-COVID19 Trial
CTID: null
Phase: Phase 3 Status: Ongoing
Date: 2020-04-09
Dexamethasone associated with hydroxychloroquine vs. hydroxychloroquine alone for the early treatment of severe ARDS caused by COVID-19 : a randomized controlled trial
CTID: null
Phase: Phase 3 Status: Prematurely Ended
Date: 2020-04-09
Hydroxychloroquine efficacy in preventing SARS-CoV-2 infection and CoVid-19 disease severity during pregnancy
CTID: null
Phase: Phase 4 Status: Ongoing
Date: 2020-04-08
Randomized open label trial assessing efficacy and safety of hydroxychloroquine plus azithromycin versus hydroxychloroquine for hospitalized patients with COVID-19
CTID: null
Phase: Phase 3 Status: Prematurely Ended
Date: 2020-04-07
Randomized clinical trial to evaluate the efficacy of hydroxychloroquine associated or not with azithromycin as a treatment for COVID-19 infection.
CTID: null
Phase: Phase 3 Status: Prematurely Ended
Date: 2020-04-07
Chemoprophylaxis of SARS-CoV-2 infection (COVID-19) in exposed healthcare workers: a randomized double-blind placebo-controlled clinical trial
CTID: null
Phase: Phase 3 Status: Completed
Date: 2020-04-07
PRE-EXPOSURE PROPHYLAXIS WITH HYDROXYCHLOROQUINE FOR HIGH-RISK HEALTHCARE WORKERS DURING THE COVID-19 PANDEMIC (PrEP_COVID): A UNICENTRIC, DOUBLE-BLINDED RANDOMIZED CONTROLLED TRIAL.
CTID: null
Phase: Phase 3 Status: Ongoing
Date: 2020-04-03
COVID-19 - Epidemiology of SARS-CoV-2 and Mortality to Covid19 Disease upon Hydroxychloroquine and Azithromycin Therapy in French Cancer patients
CTID: null
Phase: Phase 2 Status: Completed
Date: 2020-04-03
Proactive Prophylaxis with Azithromycin and Hydroxychloroquine Patients Hospitalized with COVID
CTID: null
Phase: Phase 2 Status: Prematurely Ended
Date: 2020-04-03
Monocentric Population Pharmacokinetic study of Hydroxychloroquine in COVID-19 Patients to inform its dosing optimization.
CTID: null
Phase: Phase 4 Status: Completed
Date: 2020-03-31
Evaluation of the concentration/viral effect relationship of hydroxychloroquine in COVID-19 patients in the intensive care unit.
CTID: null
Phase: Phase 4 Status: Prematurely Ended
Date: 2020-03-30
Prevention of SARS-CoV-2 (COVID-19) through Pre-Exposure Prophylaxis with Tenof
e.querySelector("font strong").innerText = 'View More'
} else if(up_display === 'none' || up_display === '') {
icon_angle_down.style.displa