| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 体外研究 (In Vitro) |
伊达比星在 MCF-7 单层细胞中的半衰期 (IC50) 为 3.3 ± 0.4 ng/mL,但在多细胞球体中为 7.9 ± 1.1 ng/mL [1]。在许多体外系统中,发现伊达比星比柔红霉素或多柔比星具有更强的细胞毒性。这是因为伊达比星具有触发拓扑异构酶 II 介导的 DNA 断裂形成的卓越能力 [2]。伊达比星的活性大约比表柔比星和阿霉素高 25 倍,分别为 57.5 倍 [3]。伊达比星的 IC50 约为 0.01 μM,以浓度依赖性方式抑制 MCF-7 细胞增殖。 Idarubicin 以浓度依赖性以及时间和浓度依赖性方式抑制 c-myc 表达 [4]。
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The drug is eliminated predominately by biliary and to a lesser extent by renal excretion, mostly in the form of idarubicinol. Biological Half-Life 22 hours |
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
97% |
| 参考文献 | |
| 其他信息 |
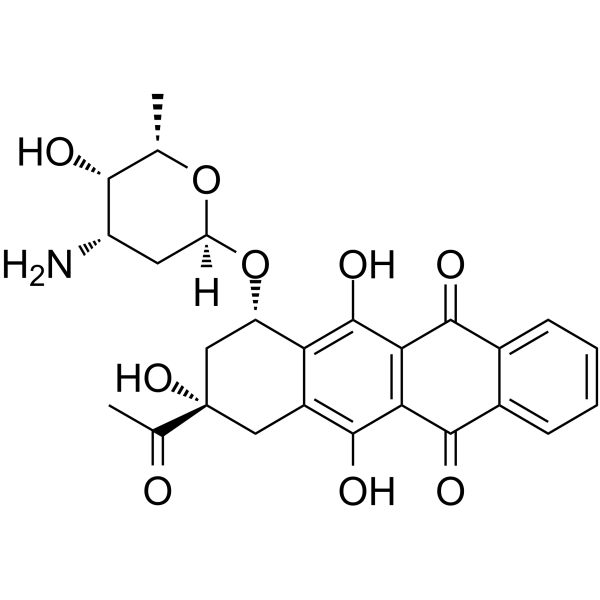
Idarubicin is a monosaccharide derivative, an anthracycline antibiotic and a deoxy hexoside. It derives from a hydride of a tetracene.
An orally administered anthracycline antineoplastic. The compound has shown activity against breast cancer, lymphomas and leukemias, together with the potential for reduced cardiac toxicity. Idarubicin is an Anthracycline Topoisomerase Inhibitor. The mechanism of action of idarubicin is as a Topoisomerase Inhibitor. Idarubicin is a semisynthetic 4-demethoxy analogue of the antineoplastic anthracycline antibiotic daunorubicin. Idarubicin intercalates into DNA and interferes with the activity of topoisomerase II, thereby inhibiting DNA replication, RNA transcription and protein synthesis. Due to its high lipophilicity, idarubicin penetrates cell membranes more efficiently than other anthracycline antibiotic compounds. An orally administered anthracycline antineoplastic. The compound has shown activity against BREAST NEOPLASMS; LYMPHOMA; and LEUKEMIA. See also: Idarubicin Hydrochloride (has salt form). Drug Indication For the treatment of acute myeloid leukemia (AML) in adults. This includes French-American-British (FAB) classifications M1 through M7. Mechanism of Action Idarubicin has antimitotic and cytotoxic activity through a number of proposed mechanisms of action: Idarubicin forms complexes with DNA by intercalation between base pairs, and it inhibits topoisomerase II activity by stabilizing the DNA-topoisomerase II complex, preventing the religation portion of the ligation-religation reaction that topoisomerase II catalyzes. Pharmacodynamics Idarubicin is an antineoplastic in the anthracycline class. General properties of drugs in this class include: interaction with DNA in a variety of different ways including intercalation (squeezing between the base pairs), DNA strand breakage and inhibition with the enzyme topoisomerase II. Most of these compounds have been isolated from natural sources and antibiotics. However, they lack the specificity of the antimicrobial antibiotics and thus produce significant toxicity. The anthracyclines are among the most important antitumor drugs available. Doxorubicin is widely used for the treatment of several solid tumors while daunorubicin and idarubicin are used exclusively for the treatment of leukemia. Idarubicin may also inhibit polymerase activity, affect regulation of gene expression, and produce free radical damage to DNA. Idarubicin possesses an antitumor effect against a wide spectrum of tumors, either grafted or spontaneous. The anthracyclines are cell cycle-nonspecific. |
| 分子式 |
C26H27NO9
|
|---|---|
| 精确质量 |
497.168
|
| CAS号 |
58957-92-9
|
| 相关CAS号 |
Idarubicin hydrochloride;57852-57-0
|
| PubChem CID |
42890
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.6±0.1 g/cm3
|
| 沸点 |
725.4±60.0 °C at 760 mmHg
|
| 闪点 |
392.5±32.9 °C
|
| 蒸汽压 |
0.0±2.5 mmHg at 25°C
|
| 折射率 |
1.706
|
| LogP |
2.95
|
| tPSA |
176.61
|
| 氢键供体(HBD)数目 |
5
|
| 氢键受体(HBA)数目 |
10
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
36
|
| 分子复杂度/Complexity |
912
|
| 定义原子立体中心数目 |
6
|
| SMILES |
C[C@H]1[C@H]([C@H](C[C@@H](O1)O[C@H]2C[C@@](CC3=C(C4=C(C(=O)C5=CC=CC=C5C4=O)C(=C32)O)O)(C(=O)C)O)N)O
|
| InChi Key |
XDXDZDZNSLXDNA-TZNDIEGXSA-N
|
| InChi Code |
InChI=1S/C26H27NO9/c1-10-21(29)15(27)7-17(35-10)36-16-9-26(34,11(2)28)8-14-18(16)25(33)20-19(24(14)32)22(30)12-5-3-4-6-13(12)23(20)31/h3-6,10,15-17,21,29,32-34H,7-9,27H2,1-2H3/t10-,15-,16-,17-,21+,26-/m0/s1
|
| 化学名 |
(7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-dione
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
A Study of ASP2215 in Combination With Induction and Consolidation Chemotherapy in Patients With Newly Diagnosed Acute Myeloid Leukemia
CTID: NCT02236013
Phase: Phase 1 Status: Completed
Date: 2024-11-05