| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 体外研究 (In Vitro) |
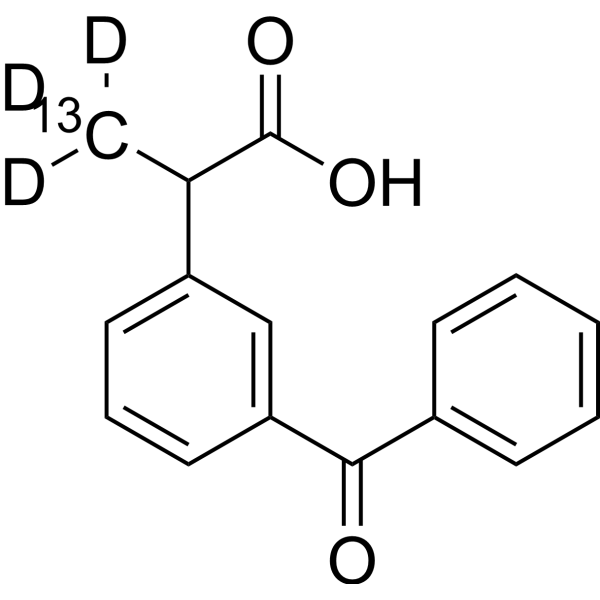
药物化合物包括碳、氢和其他元素的稳定重同位素,在药物开发过程中主要作为定量示踪剂。由于其对药物药代动力学和代谢特性的潜在影响,氘化引起了人们的兴趣[1][2]。
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Ketoprofen is rapidly and well-absorbed orally, with peak plasma levels occurring within 0.5 to 2 hours. In a 24 hour period, approximately 80% of an administered dose of ketoprofen is excreted in the urine, primarily as the glucuronide metabolite. Oral-dose cl=6.9 +/- 0.8 L/h [Ketoprofen Immediate-release capsules (4 × 50 mg)] Oral-dose cl=6.8 +/- 1.8 L/h [Ketoprofen Extended-release capsules (1 × 200 mg)] 0.08 L/kg/h 0.7 L/kg/h [alcoholic cirrhosis patients] Metabolism / Metabolites Rapidly and extensively metabolized in the liver, primarily via conjugation to glucuronic acid. No active metabolites have been identified. Ketoprofen has known human metabolites that include Ketoprofen glucuronide. Biological Half-Life Conventional capsules: 1.1-4 hours Extended release capsules: 5.4 hours due to delayed absorption (intrinsic clearance is same as conventional capsules) |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Prospective studies show that 1% to 2% of patients taking ketoprofen experience at least transient serum aminotransferase elevations. These may resolve even with drug continuation. Marked aminotransferase elevations (>3 fold elevated) occur in Likelihood score: C (probable rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Although ketoprofen has low levels in breastmilk, one center reported that they had received reports of adverse renal and gastrointestinal side effects in breastfed infants whose mothers were taking ketoprofen. Other agents are preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants All adverse reactions in breastfed infants reported in France between January 1985 and June 2011 were compiled by a French pharmacovigilance center. Of 174 reports, ketoprofen was reported to cause adverse reactions in 8 infants and to be one of the drugs most often suspected in serious adverse reactions, such as esophageal ulceration, erosive gastritis, meningeal hemorrhage, and renal insufficiency. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding 99% bound, primarily to albumin |
| 参考文献 | |
| 其他信息 |
Ketoprofen is an oxo monocarboxylic acid that consists of propionic acid substituted by a 3-benzoylphenyl group at position 2. It has a role as a non-steroidal anti-inflammatory drug, an antipyretic, an EC 1.14.99.1 (prostaglandin-endoperoxide synthase) inhibitor, an environmental contaminant, a xenobiotic and a drug allergen. It is a member of benzophenones and an oxo monocarboxylic acid. It is functionally related to a propionic acid.
Ketoprofen, a propionic acid derivative, is a nonsteroidal anti-inflammatory agent (NSAIA) with analgesic and antipyretic properties. Ketoprofen is a Nonsteroidal Anti-inflammatory Drug. The mechanism of action of ketoprofen is as a Cyclooxygenase Inhibitor. Ketoprofen is a nonsteroidal antiinflammatory drug (NSAID) used in treatment of acute pain and chronic arthritis. Ketoprofen has been linked to a low rate of serum enzyme elevations during therapy and to rare instances of clinically apparent acute liver injury. Ketoprofen has been reported in Homo sapiens with data available. Ketoprofen is a propionic acid derivate and nonsteroidal anti-inflammatory drug (NSAID) with anti-inflammatory, analgesic and antipyretic effects. Ketoprofen inhibits the activity of the enzymes cyclo-oxygenase I and II, resulting in a decreased formation of precursors of prostaglandins and thromboxanes. The resulting decrease in prostaglandin synthesis, by prostaglandin synthase, is responsible for the therapeutic effects of ibuprofen. Ketoprofen also causes a decrease in the formation of thromboxane A2 synthesis, by thromboxane synthase, thereby inhibiting platelet aggregation. An IBUPROFEN-type anti-inflammatory analgesic and antipyretic. It is used in the treatment of rheumatoid arthritis and osteoarthritis. See also: Ketoprofen lysine (is active moiety of); Ketoprofen sodium (is active moiety of); Ketoprofen; Tulathromycin (component of) ... View More ... Drug Indication For symptomatic treatment of acute and chronic rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, primary dysmenorrhea and mild to moderate pain associated with musculotendinous trauma (sprains and strains), postoperative (including dental surgery) or postpartum pain. FDA Label Treatment of musculoskeletal and connective tissue pain Mechanism of Action The anti-inflammatory effects of ketoprofen are believed to be due to inhibition cylooxygenase-2 (COX-2), an enzyme involved in prostaglandin synthesis via the arachidonic acid pathway. This results in decreased levels of prostaglandins that mediate pain, fever and inflammation. Ketoprofen is a non-specific cyclooxygenase inhibitor and inhibition of COX-1 is thought to confer some of its side effects, such as GI upset and ulceration. Ketoprofen is thought to have anti-bradykinin activity, as well as lysosomal membrane-stabilizing action. Antipyretic effects may be due to action on the hypothalamus, resulting in an increased peripheral blood flow, vasodilation, and subsequent heat dissipation. Pharmacodynamics Ketoprofen is a nonsteroidal anti-inflammatory agent (NSAIA) with analgesic and antipyretic properties. Ketoprofen has pharmacologic actions similar to those of other prototypical NSAIDs, which inhibit prostaglandin synthesis. Ketoprofen is used to treat rheumatoid arthritis, osteoarthritis, dysmenorrhea, and alleviate moderate pain. |
| 分子式 |
C16H14O3
|
|---|---|
| 分子量 |
258.291704654694
|
| 精确质量 |
258.116
|
| CAS号 |
1189508-77-7
|
| 相关CAS号 |
Ketoprofen;22071-15-4
|
| PubChem CID |
3825
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| LogP |
3.105
|
| tPSA |
54.37
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
19
|
| 分子复杂度/Complexity |
331
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CC(C1=CC(=CC=C1)C(=O)C2=CC=CC=C2)C(=O)O
|
| InChi Key |
DKYWVDODHFEZIM-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C16H14O3/c1-11(16(18)19)13-8-5-9-14(10-13)15(17)12-6-3-2-4-7-12/h2-11H,1H3,(H,18,19)
|
| 化学名 |
2-(3-benzoylphenyl)propanoic acid
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.8716 mL | 19.3581 mL | 38.7162 mL | |
| 5 mM | 0.7743 mL | 3.8716 mL | 7.7432 mL | |
| 10 mM | 0.3872 mL | 1.9358 mL | 3.8716 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Characterization of Treatment Responses in Lymphedema
CTID: NCT03783715
Phase: Status: Terminated
Date: 2023-10-10