| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| Other Sizes |
|
| 靶点 |
Orexin (hypocretin) receptor
|
|---|---|
| 体外研究 (In Vitro) |
Orexin (hypocretin) 神经肽与觉醒/睡眠控制有关,之前已经证明拮抗食欲素信号通路可以促进动物和人类的睡眠。这一机制开辟了一种新的治疗方法,可以抑制失眠患者的过度觉醒,而不是促进与睡眠相关的信号传导。在这里,我们描述了双食欲素受体拮抗剂(DORA)lemborexant((1R,2S)-2-{[(2,4-二甲基嘧啶-5-基)氧基]甲基}-2-(3-氟苯基)-N-(5-氟吡啶-2-基)环丙烷甲酰胺)的临床前体外和计算机药理学特征,它是一种用于失眠症状治疗的新型实验性治疗剂,并将其特性与其他两种DORA,即阿米氧嘧啶和suvorexant进行了比较。Lemborexant与食欲素受体结合,并以低纳摩尔效力的竞争方式在功能上抑制它们,在人类、大鼠和小鼠受体之间没有明显的物种差异。两种食欲素受体的结合和解离动力学都很快。Lemborexant对食欲素受体的选择性超过了其他88种具有重要生理功能的受体、转运蛋白和离子通道。将lemborexant模拟为食欲素受体的计算机模拟表明,它在受体结合袋内与suvorexant具有相同类型的构象,即π堆叠的马蹄形构象[2]。
|
| 体内研究 (In Vivo) |
Lemborexant阻止了食欲素促进的大鼠促肾上腺皮质激素的增加,因此证明了食欲素信号通路的抑制。此外,lemborexant促进了野生型小鼠和大鼠的睡眠。Lemborexant以相等的速率促进REM和非REM睡眠(REM睡眠比率没有变化)。相比之下,唑吡坦减少了REM睡眠。lemborexant的睡眠促进作用是通过食欲素肽信号通路介导的,食欲素神经元缺陷小鼠缺乏睡眠促进作用就证明了这一点。长期给药与给药后即刻效应大小或睡眠结构的变化无关。Lemborexant不会增加乙醇的镇静作用或损害运动协调,在动物身上显示出良好的安全裕度。小鼠和大鼠的药代动力学/药效学数据非常一致[1]。
|
| 酶活实验 |
通过受体结合试验测量亲和力[2]
使用96孔Flashplate通过受体结合分析(RBA)测定结合亲和力。膜组分由表达人OX1R(hOX1R)或人OX2R(hOX2R)的中国仓鼠卵巢(CHO)细胞制备。将hOX1R或hOX2R(5µg蛋白质/测定)的膜悬浮液与测试拮抗剂[lemborexant(0.6-200 nmol/l)、almorexant(0.2-200 nmol/l)或suvorexant(0.2–60 nmol/1)]以及OXA(10µmol/l)溶液或载体和[125I]OXA溶液(0.2 nmol/l)混合。将混合物(最终体积为100µl)在96孔闪存盘上于室温下孵育30分钟。丢弃所有反应混合物,然后用200µl含有525 mmol/l NaCl的25 mmol/l HEPES缓冲液进行两次洗涤。通过TopCount测量每个孔的剩余放射性(以dpm为单位),并使用以下公式计算试验拮抗剂的抑制活性: 其中,在存在试验拮抗剂(试验)的情况下,T以dpm表示,在存在10µmol/l OXA(非特异性结合)的条件下,N以dpm表达,在不存在化合物(对照)的情况中,C以dpm表述。 实验中的值分为三份(lemborexant、almorexant)或四份(suvorexant)。以相同的方式进行了三次lemborexant实验,在计算最终IC50值及其S.E.M.的平均值之前,计算了每个实验的IC50值。almorexant和suvorexant的实验进行了一次,每个值表示为平均值±S.E.M.进行统计分析。 |
| 细胞实验 |
基于细胞的功能报告酶测定[1]
HEK293细胞用人或小鼠OX1R或OX2R以及报告系统(Chen等人,1995;Durocher等人,2000)稳定转染,其中报告酶[胎盘碱性磷酸酶(PLAP)](Goto等人,1996)可以通过细胞内Ca2+依赖性报告单元在功能性OXR激活后诱导。 将细胞以10000/孔的密度接种到96孔板中,并在培养基中培养过夜。第二天,将5µl的lemborexant溶液加入96孔板中的培养细胞中,使最终培养基体积为115µl(23倍稀释),从而得到1、3、10、30、100、300和1000 nmol/l的终浓度,用于细胞培养。 在加入lemborexant并在室温下孵育约2-3小时后,将食欲素肽激动剂人/小鼠OXA、人OXB(hOXB)、小鼠OXB(mOXB)或修饰的[Ala11,d-Leu15]-OXB在Dulbecco的修饰Eagle培养基(含0.1%牛血清白蛋白和3.45µmol/l福司可林)中稀释,并将10µl加入细胞孔中,得到115-µl的最终体积。肽激动剂的最终浓度范围为0.01至1000 nmol/l。搅拌平板混合后,将细胞在37°C下孵育约20小时,将lemborexant和肽激动剂各自的浓度组合应用于四个细胞孔。hOXB和mOXB之间有两种不同的氨基酸。因此,hOX2R被hOXB激活,小鼠OX2R(mOX2R)被mOXB激活。[Ala11,d-Leu15]-OXB已被描述为对OX2R的选择性高于天然OXB(Asahi等人,2003)。 |
| 动物实验 |
Rodent (wild-type rats and wild-type and orexin neuron-deficient [orexin/ataxin-3 Tg/+] mice) studies were performed to evaluate the effects of single-dose oral lemborexant (1–300 mg/kg) on orexin-induced increases in plasma adrenocorticotropic hormone (ACTH), locomotor activity, vigilance state measures (wakefulness, nonrapid eye movement [non-REM] sleep, rapid eye movement [REM] sleep), ethanol-induced anesthesia, and motor coordination, and the effects of multiple-dose oral lemborexant (30 mg/kg) on vigilance state measures. Active comparators were almorexant and zolpidem. Pharmacokinetics were assessed after single-dose lemborexant in mice and rats.[1]
lemborexant, almorexant, and zolpidem were suspended as free bases in the vehicle solution specified for each study. Doses for each compound in the mouse experiments were set based on the minimum necessary dose for sleep promotion in mice, and are therefore different for each compound.[1] Effect of single-dose lemborexant on the orexin-induced increase in ACTH in rats.[1] Male F344 rats (age: 5 weeks; body weight: 85.8–103.5 g) were implanted with infusion cannulae into the left lateral ventricle for intracerebroventricular (i.c.v.) injection. Four to five days after surgery, rats were habituated for oral administration (p.o.) and handling once before the study. Six to seven days after cannula implantation, rats received p.o. vehicle (5% [v/v] dimethyl sulfoxide, 9.5% [v/v] cremophor in saline; n = 10) or lemborexant (5 mL/kg suspended in vehicle) 1, 3, 10, or 30 mg/kg (n = 5, 6, 6, and 5, respectively). One hour later, vehicle control rats received 5 µL of phosphate buffered saline (PBS) or [Ala11, D-Leu15]-orexin B (1 nmol/head, 0.2 mmol/L in PBS) via i.c.v. injection (n = 5 each). All lemborexant pretreated rats received [Ala11, D-Leu15]-orexin B via i.c.v. injection. Fifteen minutes later, rats were decapitated and blood samples were collected with Na2EDTA (100 mg/mL, 100 µL). Blood samples were then centrifuged (1000 × g, 10 min at 4°C) and supernatant plasma was stored at −80°C for later measurement of ACTH and lemborexant concentrations. After decapitation, blue ink was injected i.c.v. to confirm the correct placement of cannulae. Coronal cross-sections were made near the cannulae; placement was judged (by an experienced observer) to be correct if blue ink was visible in the lateral ventricle(s). Data from 3 out of 32 rats with incorrect cannula placement were excluded from analysis. Effect of single-dose lemborexant on spontaneous locomotor activity in wild-type and orexin neuron-deficient mice[1] Wild-type male mice (age: 9 weeks; body weight: 19.4–22.5 g) were dosed p.o. with vehicle (5% [v/v] dimethyl sulfoxide, 10% [v/v] cremophor in 10 mmol/L HCl; 10 mL/kg; n = 16) or lemborexant (30 [n = 8] or 100 mg/kg [n = 7]) at Zeitgeber time 3:40 or 5:30. One hour after dosing, mice were placed in an open field arena and locomotor activity was automatically recorded as infrared light beam breaks as previously described. For activity values, all horizontal and vertical infrared light beam break counts were summed over 1 h after the start of locomotor activity recording.[1] In a separate study, orexin neuron-deficient mice (age: 18–26 weeks; body weight: 27.9–36.0 g) were dosed p.o. with vehicle (as above; n = 8) or 100 mg/kg lemborexant (n = 8), the maximum dose tested in wild-type mice, at Zeitgeber time 3:40 or 5:30. Thirty minutes later, locomotor activity was recorded and summed over 1 h as described above. View More
Effect of single-dose lemborexant on vigilance state measures in wild-type and orexin neuron-deficient mice[1] Effect of single-dose lemborexant on vigilance state measures in rats[1] Under deep sodium pentobarbital anesthesia, male Sprague Dawley rats (age: 7–10 weeks; body weight: 311–449 g) were intraperitoneally implanted with battery-driven, wireless telemetry devices (TL11M2-F40-EET) for EEG/EMG measurements. Two silver screws were fixed 2.0 mm left and right of lambda through the skull bone so as to touch the dura mater. EEG leads were knotted to the screws, while EMG leads were placed intranuchally into pockets formed by blunt dissection of neck muscle left and right of midline. After 9–11 days of recovery in home cages, rats were habituated to the recording room and p.o. dosing procedure for 2 days, while still being housed in the same home cages throughout the experiment. A total of 12 rats were then selected for the study and assigned to body-weight matched groups for five dosing and recording sessions. The sessions were conducted with 2–3 days of intermittent wash-out periods, with p.o. dosing taking place at Zeitgeber time 2:00–3:00 and subsequent recording of EEG/EMG signals for 4 h using a telemetry system for later off-line analysis. Continuous recordings were divided into 10-s epochs and automatically analyzed via an algorithm that determined vigilance states. Automated analysis results were then verified and, if necessary, corrected by a trained observer blinded to treatment. No animal received the same test compound at the same dose twice. There were a total of 10 treatment groups in the study; vehicle (10 mL/kg 0.5% [w/v] methyl-cellulose 400 [n = 6]), lemborexant (3, 10, 30, 100, or 300 mg/kg [all n = 6]), and zolpidem (3, 10, 30, or 100 mg/kg [all n = 6]). Effect of chronic-dose lemborexant on vigilance state measures in rats[1] Male Sprague Dawley rats, fully habituated to experimental conditions from the previously described single-dose study, were used after a 5-day washout period. All rats were dosed p.o., once-daily at Zeitgeber time 2:00–3:00, with vehicle (0.5% [w/v] methylcellulose 400) on day 1–3, then vehicle (n = 2), lemborexant 30 mg/kg (n = 5), or zolpidem 100 mg/kg (n = 5) from day 4 to 24, and finally vehicle on days 25 and 26. EEG/EMG signals were recorded (as already described for the single-dose study) on days 1 and 2 (pretreatment), days 4, 7, 11, 14, 18, 21, and 24 (treatment), and days 25 and 26 (posttreatment) for nearly 3 hours. Lemborexant and zolpidem doses were chosen based on the maximum effects in the previous single-dose experiment. Effect of single-dose lemborexant on ethanol-induced anesthesia in wild-type mice[1] Wild-type male mice (age: 13 weeks; body weight: 21.8–28.1 g) were dosed p.o. with vehicle (10 mL/kg 0.5% [w/v] methylcellulose 400), lemborexant (1, 3, or 10 mg/kg), almorexant (30, 100, or 300 mg/kg), or zolpidem (3, 10, or 30 mg/kg) (all groups, n = 6) during the light phase. After 5 min, mice received intraperitoneal injections of 3.0 g/kg ethanol (20% [w/v] in saline). The almorexant and zolpidem doses were based on those used in a previous rat study, where almorexant up to 300 mg/kg did not show interaction with ethanol, but zolpidem from 10 mg/kg upwards showed interaction with ethanol. Effect of single-dose lemborexant on motor coordination in wild-type mice [1] For this study, male wild-type mice (age: 14 weeks; body weight: 22.1–29.1 g), previously trained on the treadmill for 3 consecutive days with subsequent 14 days rest, were allocated to body-weight equivalent groups to receive single p.o. dosing of vehicle (10 mL/kg 0.5% [w/v] methylcellulose 400), lemborexant (30, 100, or 300 mg/kg), or zolpidem (100 mg/kg) (all groups, n = 11). The starting lemborexant dose (30 mg/kg) was selected as this is approximately threefold the sleep-promoting dose, while zolpidem 100 mg/kg has previously been reported to impair motor coordination in rats Plasma and CSF concentrations of lemborexant after single dosing in rats[1] Male Sprague Dawley rats (age: 9 weeks; body weight: 337–355 g) were dosed p.o. with lemborexant 30 mg/kg in 0.5% (w/v) methylcellulose 400 (5 mL/kg) at Zeitgeber time 4:30–5:00. Two hours later, rats were anesthetized and plasma and CSF samples were obtained from the abdominal aorta and cisterna magna, respectively, for the measurement of lemborexant concentrations by LC-MS/MS. Plasma concentrations of lemborexant after single dosing in mice[1] Male C57BL/6N mice (age: 14 weeks; body weight: 26.7–31.7 g) were dosed p.o. with lemborexant 10 or 300 mg/kg in 0.5% [w/v] methylcellulose 400 (10 mL/kg) at Zeitgeber time 3:00–9:15. At 0.25, 0.5, 1, 3, 5, 6, 18, and 24 h after dosing, mice were anesthetized and plasma samples were obtained from the abdominal aorta for measurement of lemborexant concentrations by LC-MS/MS. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Animal models of lemborexant disposition have demonstrated rapid absorption following oral administration. The Tmax of lemborexant is approximately 1-3 hours, or 3-5 hours following administration of a high-fat, high-calorie meal. Cmax and AUC0-24h increase at a rate slightly less than proportionate to the given dose. Following administration of a high-fat, high-calorie meal, Cmax is decreased by 23% and AUC0-inf is increased by 18%. AUC, Cmax, and terminal half-life are increased in the presence of moderate hepatic impairment, and AUC (but not half-life) is increased in the presence of mild hepatic impairment. Following oral administration, 57.4% of the dose is found in the feces and 29.1% in the urine. Less than 1% of the dose recovered in the urine exists as unchanged parent drug, suggesting extensive metabolism. The volume of distribution of lemborexant is 1970 L, indicating extensive tissue distribution. Metabolism / Metabolites Given that less than 1% of an administered dose is recovered unchanged in the urine, it is likely that lemborexant is extensively metabolized - this has been confirmed in rat and monkey models, but its metabolism in humans has not been fully characterized. Prescribing information states that it is predominantly metabolized by CYP3A4, with a smaller contribution by CYP3A5. The major circulating metabolite is lemborexant's M10 metabolite, which is pharmacologically active and binds to orexin receptors with a similar affinity to the parent drug. The M10 metabolite has the potential to induce CYP3A and CYP2B6 enzymes, weakly inhibit CYP3A enzymes, and is a substrate of P-gp transporters. Biological Half-Life The half-life for lemborexant at doses of 5mg and 10mg is 17 and 19 hours, respectively. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In several clinical trials, lemborexant was found to be well tolerated, with serum ALT elevations above 3 times the upper limit of normal in less than 1% of treated subjects and with similar rates in placebo recipients. The elevations were transient and asymptomatic, none required dose modification or discontinuation, and none were associated with a simultaneous elevation in serum bilirubin. Thus, in the registration trials of lemborexant, there were no reports of clinically apparent liver injury. Lemborexant has been available for a limited period of time, but has yet to be implicated in causing clinically apparent liver injury with its more widespread clinical use. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Amounts of lemborexant in milk appear to be low. If lemborexant is required by the mother, it is not a reason to discontinue breastfeeding. However, until more data become available, monitor the infant for sedation, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Lemborexant is approximately 94% protein-bound _in vitro_, though the specific proteins to which it binds in plasma have not been elucidated. |
| 参考文献 |
|
| 其他信息 |
Lemborexant is a DEA Schedule IV controlled substance. Substances in the DEA Schedule IV have a low potential for abuse relative to substances in Schedule III. It is a Depressants substance.
Lemborexant is a novel dual orexin receptor antagonist used in the treatment of insomnia characterized by difficulties with sleep onset and/or sleep maintenance. Recent research in the field of sleep disorders has revealed that insomnia is likely driven not by the inability of the brain to "switch on" sleep-related circuits, but rather an inability to "switch-off" wake-promoting circuits. Whereas historically popular pharmacologic treatments for insomnia (e.g. [zopiclone], [zolpidem], benzodiazepines) focus on enhancing sleep drive via modulation of GABA and melatonin receptors, lemborexant and other orexin antagonists (e.g. [suvorexant]) act to counteract inappropriate wakefulness. This novel mechanism of action offers potential advantages over classic hypnotic agents, including a more favorable adverse effect profile and potentially greater efficacy, and may signal the beginning of a new wave of treatment options for patients suffering from insomnia. Lemborexant is an Orexin Receptor Antagonist. The mechanism of action of lemborexant is as an Orexin Receptor Antagonist, and Cytochrome P450 2B6 Inducer. Lemborexant is an orexin receptor antagonist used for the treatment of insomnia and sleep disorders. Lemborexant therapy is associated with rare occurrence of transient serum enzyme elevations, but has not been implicated in cases of clinically apparent liver injury. Drug Indication Lemborexant is indicated for the treatment of adult patients with insomnia characterized by difficulties with sleep onset and/or sleep maintenance. FDA Label Mechanism of Action The orexin neuropeptide signaling system is involved in many physiologic functions, including sleep/wake control. Orexin-A and orexin-B activate post-synaptic G-protein coupled orexin-1 receptors (OX1R) and orexin-2 receptors (OX2R), which are found on neurons in the hypothalamus that project to numerous wake-controlling nuclei. Each receptor carries slightly different activity - activation of OX1R appears to suppress the onset of rapid eye movement (REM) sleep, whereas activation of OX2R appears to suppress non-REM sleep. Lemborexant is an competitive antagonist of OX1R and OX2R. By blocking the binding of wake-promoting orexin-A and -B at these receptors, lemborexant suppresses the wake-drive, thereby promoting sleep. Pharmacodynamics Lemborexant promotes sleep by antagonizing the actions of wake-promoting chemicals in the brain. Episodes of complex sleep behaviors (e.g. eating food, having sex, making phone calls) have been reported in patients using lemborexant - these events may occur in hypnotic-naive and hyponotic-experienced patients, and patients are unlikely to remember these events. Patients exhibiting complex sleep behaviors should discontinue lemborexant immediately. Lemborexant may carry some risk of abuse, and should be used with caution in patients with a history of alcohol or drug addiction. Its controlled substance schedule is currently under review by the Drug Enforcement Administration. |
| 分子式 |
C22H20F2N4O2
|
|---|---|
| 分子量 |
410.4248
|
| 精确质量 |
410.155
|
| CAS号 |
1369764-02-2
|
| 相关CAS号 |
1369764-02-2
|
| PubChem CID |
56944144
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
596.1±50.0 °C at 760 mmHg
|
| 闪点 |
314.3±30.1 °C
|
| 蒸汽压 |
0.0±1.7 mmHg at 25°C
|
| 折射率 |
1.619
|
| LogP |
3.16
|
| tPSA |
77
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
30
|
| 分子复杂度/Complexity |
612
|
| 定义原子立体中心数目 |
2
|
| SMILES |
O=C([C@H]1[C@@](C2=CC=CC(F)=C2)(COC3=CN=C(C)N=C3C)C1)NC4=NC=C(F)C=C4
|
| InChi Key |
MUGXRYIUWFITCP-PGRDOPGGSA-N
|
| InChi Code |
InChI=1S/C22H20F2N4O2/c1-13-19(11-25-14(2)27-13)30-12-22(15-4-3-5-16(23)8-15)9-18(22)21(29)28-20-7-6-17(24)10-26-20/h3-8,10-11,18H,9,12H2,1-2H3,(H,26,28,29)/t18-,22+/m0/s1
|
| 化学名 |
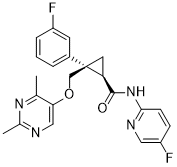
(1R,2S)-2-[(2,4-dimethylpyrimidin-5-yl)oxymethyl]-2-(3-fluorophenyl)-N-(5-fluoropyridin-2-yl)cyclopropane-1-carboxamide
|
| 别名 |
E-2006; Dayvigo; E 2006; 1369764-02-2; Dayvigo; UNII-0K5743G68X; 0K5743G68X; (1R,2S)-2-[(2,4-dimethylpyrimidin-5-yl)oxymethyl]-2-(3-fluorophenyl)-N-(5-fluoropyridin-2-yl)cyclopropane-1-carboxamide; E2006; Lemborexant
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4365 mL | 12.1826 mL | 24.3653 mL | |
| 5 mM | 0.4873 mL | 2.4365 mL | 4.8731 mL | |
| 10 mM | 0.2437 mL | 1.2183 mL | 2.4365 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05594589 | Recruiting | Drug: Lemborexant Other: PBO |
Sleep Initiation and Maintenance Disorders |
Eisai Co., Ltd. | November 30, 2022 | Phase 2 |
| NCT05463861 | Recruiting | Drug: Lemborexant Drug: Placebo |
Delayed Sleep Phase Syndrome | Stanford University | February 1, 2022 | Phase 4 |
| NCT05344443 | Recruiting | Drug: Lemborexant Drug: Oral Placebo |
Shift-Work Related Sleep Disturbance |
University of California, San Francisco |
March 10, 2022 | Phase 4 |
| NCT05763329 | Recruiting | Drug: Placebo Day 1 Drug: Placebo Day 2 |
OSA | Chulalongkorn University | February 1, 2023 | Phase 1 Phase 2 |
| NCT06093126 | Not yet recruiting | Drug: Lemborexant 5 MG | Insomnia Dementia Frontotemporal Dementia |
Nova Scotia Health Authority | November 1, 2023 | Phase 4 |