| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|

| 靶点 |
Neurokinin-1 receptor
|
|---|---|
| 体外研究 (In Vitro) |
Netupitant (CID-6451149) 以高亲和力结合人类 NK1 受体 (pKi=9.0),选择性比 NK2 和 NK3 受体高 1000 倍以上(两个位点的 pKi=5.8)[2]。 Netupitant (1, 10, 100 nM) 浓度依赖性地拮抗 P 物质 (SP) 的刺激作用,在 CHO NK1 细胞中表现出难以克服的拮抗作用 (pKB=8.87)[2]。
|
| 体内研究 (In Vivo) |
Netupitant(CID-6451149;1-10 mg/kg;腹膜内注射)剂量依赖性地抑制 SP 引发的小鼠典型的抓、咬和舔反应。在沙鼠中,脑室内注射 NK1 激动剂引起的足部敲击行为会被腹腔注射 (ID50 1.5mg/kg) 或口服 (ID50 0.5mg/kg) 的 Netupitant 剂量依赖性地抵消[2]。 Netupitant (0.1-3 mg/kg; iv) 对逼尿肌中 SP-甲酯 (SP-OMe) 的反应产生浓度依赖性抑制(平均 pKB=9.24)。 Netupitant 可降低反射性膀胱收缩的频率[3]。
|
| 酶活实验 |
受体结合筛查概况/Receptor binding screening profile[2]
在表达三种人速激肽受体以及50种不同GPCR、单胺转运蛋白和离子通道的CHO细胞膜上进行的受体结合实验中评估了Netupitant。本研究通过CEREP根据合同进行。 表达人或大鼠速激肽受体的细胞中的钙动员研究[2] 稳定表达NK受体的细胞是T.Costa教授(ISS,Rome,IT,稳定表达大鼠NK1受体的HEK293细胞)、C.Rojas教授(Johns Hopkins University School of Medicine,Baltimore,US,稳定表达人NK1受体)和T.W.Schwartz教授(CHO细胞表达人NK2或NK3受体)实验室的慷慨礼物。CHO细胞在RPMI 1640培养基中维持,该培养基补充了10%胎牛血清、2 mM l-谷氨酰胺、100 U/ml青霉素和100μg/ml链霉素以及200 mg/l G418。HEK293rNK1细胞在补充有10%胎牛血清、2 mM l-谷氨酰胺、100 U/ml青霉素和100μg/ml链霉素和100 mg/l潮霉素的Eagle最低必需培养基中维持。细胞在37°C的5%CO2加湿空气中培养,以50000个细胞/孔的密度接种到96孔的黑色透明底板中。第二天,将细胞与补充有2.5 mM丙磺舒、3μM钙敏感荧光染料Fluo-4 AM和0.01%普朗尼克酸的培养基在37°C下孵育30分钟。随后,吸取加载溶液,并加入100μl/孔的Hank's平衡盐溶液(HBSS),该溶液补充了20 mM HEPES、2.5 mM丙磺舒和500μM亮黑。[2] SP、NKA、NKB、[Sar9、Met(O2)11]SP、[βAla8]NKA(4-10)和[MePhe7]NKB 1 mM溶解在双蒸馏水中。将1 mM的NK受体拮抗剂(阿普替坦、奈替坦、SR48968、GR159897、SR142801和SB222200)溶解在DMSO中。在HBSS/HEPES(20mM)缓冲液(含0.02%BSA组分V)中进行连续稀释。[2] 将两个板(细胞板和复合板)放入FlexStation II后,在37°C下测量荧光变化。在线添加的体积为50μl/孔。在拮抗剂型实验中,在加入激动剂之前,将所研究的化合物预孵育24分钟。为了促进拮抗剂型实验中药物扩散到孔中,在向孔中注射拮抗剂后立即进行了三个混合循环(每个孔25μl上下移动3次)。荧光的最大变化(以超过基线水平的百分比表示)用于测量激动剂反应。 |
| 细胞实验 |
将细胞在生长培养基(对照)或含有拮抗剂的培养基中于 37°C 预孵育一小时。为了保证受体饱和,拮抗剂浓度必须至少比 Kd 值高 30 倍。为了使仍然附着在受体上的拮抗剂解离,在预孵育后从细胞中除去拮抗剂,并仅用生长培养基冲洗细胞额外一小时。然后,将等渗 HEPES 缓冲液(pH 7.4,20 mM)添加到细胞培养基中。该缓冲液含有以下浓度的 SP 和 NaCl:3 nM 至 1 mM; KCl (2 mM)、MgCl2 (1 mM)、CaCl2 (2 mM)、Fluo-4 乙酰氧基甲基 (AM) 酯 (2 mM)、pluronic 酸 (0.04%) 和 MgCl2 (1 mM)。最后一次孵育在 37°C 下进行一小时。为了将 AM 酯分子隔离到胶束中以供细胞摄取,添加普朗尼克酸作为非离子表面活性剂。
|
| 动物实验 |
SP (0.01–1 nmol) was given intrathecally (i.t.). I.t. injections (5 μl per mouse) were given under light (just sufficient to produce a loss of the righting reflex) isofluorane anesthesia according to the procedure described by Hylden and routinely adopted in our laboratory. Approximately 45 min before i.t. injection, the mice were adapted to an individual plastic cage which served as the observation chamber. The animals were challenged with SP and individually observed for 10 or 15 min. The total time (s) spent by the animal displaying the following behaviors was measured: hindlimb scratching directed toward the flank; biting or licking of the fore and hind paw; and biting or licking of the tail. Netupitant and Aprepitant (1 and 10 mg/kg, i.p.) were administered 30 min before SP (0.1 nmol i.t.). All experiments were started at 9.00 am[2].
Introduction. Tachykinins potently contract the isolated urinary bladder from a number of animal species and play an important role in the regulation of the micturition reflex. On the guinea-pig isolated urinary bladder we examined the effects of a new potent and selective NK1 receptor antagonist (netupitant) on the contractions induced by a selective NK1 receptor agonist, SP-methylester (SP-OMe). Moreover, the effects of netupitant and another selective NK1 antagonist (L-733,060) were studied in anesthetized guinea-pigs using two experimental models, the isovolumetric bladder contractions and a model of bladder overactivity induced by intravesical administration of acetic acid (AA). Methods and Results. Detrusor muscle strips were mounted in 5 mL organ baths and isometric contractions to cumulative concentrations of SP-OME were recorded before and after incubation with increasing concentrations of netupitant. In anesthetized female guinea-pigs, reflex bladder activity was examined under isovolumetric conditions with the bladder distended with saline or during cystometry using intravesical infusion of AA. After a 30 min stabilization period, netupitant (0.1-3 mg/kg, i.v.) or L-733,060 (3-10 mg/kg, i.v.) were administered. In the detrusor muscle, netupitant produced a concentration-dependent inhibition (mean pKB = 9.24) of the responses to SP-OMe. Under isovolumetric conditions, netupitant or L-733,060 reduced bladder contraction frequency in a dose-dependent manner, but neither drug changed bladder contraction amplitude. In the AA model, netupitant dose-dependently increased intercontraction interval (ICI) but had no effect on the amplitude of micturition (AM). L-733,060 dose-dependently increased ICI also but this effect was paralleled by a significant reduction of AM. Conclusion. Netupitant decreases the frequency of reflex bladder contractions without altering their amplitude, suggesting that this drug targets the afferent limb of the micturition reflex circuit and therefore may be useful clinically in treating bladder overactivity symptoms.[3] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Upon oral administration of a single dose of netupitant, netupitant started to be measurable in plasma between 15 minutes and 3 hours after dosing. Plasma concentrations reached Cmax in approximately 5 hours. There was a greater than dose-proportional increase in the systemic exposure with the dose increase from 10 mg to 300 mg and a dose-proportional increase in systemic exposure with a dose increase from 300 mg to 450 mg. Primarily fecal. In cancer patients, Vz/F: 1982 ± 906 L (mean ± SD). Estimated systemic clearance of 20.3 ± 9.2 L/h (mean ± SD). Metabolism / Metabolites Once absorbed, netupitant is extensively metabolized to form three major metabolites: desmethyl derivative, M1; N-oxide derivative, M2; and OH-methyl derivative, M3. Metabolism is mediated primarily by CYP3A4 and to a lesser extent by CYP2C9 and CYP2D6. Metabolites M1, M2 and M3 were shown to bind to the substance P/neurokinin 1 (NK1) receptor. Biological Half-Life 96 hours with CV% of 61. |
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
> 99.5% at drug concentrations ranging from 10-1300 ng/mL. |
| 参考文献 |
|
| 其他信息 |
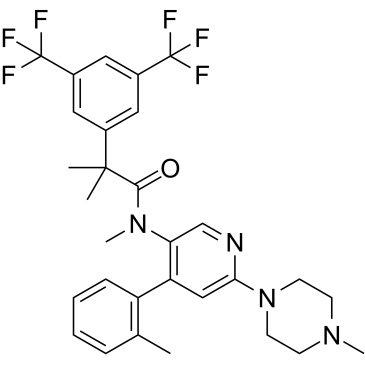
Netupitant is a monocarboxylic acid amide obtained by formal condensation of the carboxy group of 2-[3,5-bis(trifluoromethyl)phenyl]-2-methylpropanoic acid with the secondary amino group of N-methyl-4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-amine; an antiemetic used in combination with palonosetron hydrochloride (under the trade name Akynzeo) to treat nausea and vomiting in patients undergoing cancer chemotherapy. It has a role as an antiemetic and a neurokinin-1 receptor antagonist. It is a monocarboxylic acid amide, an organofluorine compound, an aminopyridine, a member of toluenes, a N-alkylpiperazine and a N-arylpiperazine.
Netupitant is an antiemitic drug approved by the FDA in October 2014 for use in combination with palonosetron for the prevention of acute and delayed vomiting and nausea associated with cancer chemotherapy including highly emetogenic chemotherapy. Netupitant is a neurokinin 1 receptor antagonist. The combination drug is marketed by Eisai Inc. and Helsinn Therapeutics (U.S.) Inc. under the brand Akynzeo. Netupitant is a Substance P/Neurokinin-1 Receptor Antagonist. The mechanism of action of netupitant is as a Neurokinin 1 Antagonist, and Cytochrome P450 3A4 Inhibitor, and P-Glycoprotein Inhibitor, and Breast Cancer Resistance Protein Inhibitor. Netupitant is a selective neurokinin 1 (NK1) receptor antagonist with potential antiemetic activity. Netupitant competitively binds to and blocks the activity of the human substance P/NK1 receptors in the central nervous system (CNS), thereby inhibiting NK1-receptor binding of the endogenous tachykinin neuropeptide substance P (SP), which may result in the prevention of chemotherapy-induced nausea and vomiting (CINV). SP is found in neurons of vagal afferent fibers innervating the brain-stem nucleus tractus solitarii and the area postrema, which contains the chemoreceptor trigger zone (CTZ), and may be elevated in response to chemotherapy. The NK-receptor is a G-protein receptor coupled to the inositol phosphate signal-transduction pathway and is found in both the nucleus tractus solitarii and the area postrema. Drug Indication Netupitant is an antiemitic drug approved by the FDA in October 2014 for use in combination with palonosetron for the prevention of acute and delayed vomiting and nausea associated with cancer chemotherapy including highly emetogenic chemotherapy. FDA Label Mechanism of Action Delayed emesis (vomiting) has been largely associated with the activation of tachykinin family neurokinin 1 (NK1) receptors (broadly distributed in the central and peripheral nervous systems) by substance P. As shown in in vitro and in vivo studies, netupitant inhibits substance P mediated responses. |
| 分子式 |
C30H32F6N4O
|
|---|---|
| 分子量 |
578.59
|
| 精确质量 |
578.248
|
| 元素分析 |
C, 62.28; H, 5.57; F, 19.70; N, 9.68; O, 2.77
|
| CAS号 |
290297-26-6
|
| 相关CAS号 |
Netupitant-d6; 2070015-31-3; Netupitant metabolite N-desmethyl Netupitant; 290296-72-9; N-desmethyl Netupitant-d6; Netupitant metabolite Netupitant N-oxide; 910808-11-6; Netupitant N-oxide-d6; Netupitant metabolite Monohydroxy Netupitant; 910808-12-7; Monohydroxy Netupitant-d6; 290296-54-7 (2HCl); 290297-26-6
|
| PubChem CID |
6451149
|
| 外观&性状 |
White solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
597.4±50.0 °C at 760 mmHg
|
| 闪点 |
315.1±30.1 °C
|
| 蒸汽压 |
0.0±1.7 mmHg at 25°C
|
| 折射率 |
1.540
|
| LogP |
6.39
|
| tPSA |
39.68
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
10
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
41
|
| 分子复杂度/Complexity |
865
|
| 定义原子立体中心数目 |
0
|
| SMILES |
FC(C1C([H])=C(C(F)(F)F)C([H])=C(C=1[H])C(C([H])([H])[H])(C([H])([H])[H])C(N(C([H])([H])[H])C1=C([H])N=C(C([H])=C1C1=C([H])C([H])=C([H])C([H])=C1C([H])([H])[H])N1C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])C1([H])[H])=O)(F)F
|
| InChi Key |
WAXQNWCZJDTGBU-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C30H32F6N4O/c1-19-8-6-7-9-23(19)24-17-26(40-12-10-38(4)11-13-40)37-18-25(24)39(5)27(41)28(2,3)20-14-21(29(31,32)33)16-22(15-20)30(34,35)36/h6-9,14-18H,10-13H2,1-5H3
|
| 化学名 |
2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl]propanamide
|
| 别名 |
AGE-94200; Ro67-3189; AGE 94200; Ro 67-3189/000; AGE94200; Ro 67-3189; Ro-67-3189; 2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl]propanamide; 2-(3,5-bis(trifluoromethyl)phenyl)-N,2-dimethyl-N-(6-(4-methylpiperazin-1-yl)-4-(o-tolyl)pyridin-3-yl)propanamide; Ro-67-3189; Ro-673189000; Ro 67-3189/000; CHEMBL206253; Netupitant
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 2~9.1 mg/mL (3.5~15.71 mM)
Ethanol: ~100 mg/mL |
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7283 mL | 8.6417 mL | 17.2834 mL | |
| 5 mM | 0.3457 mL | 1.7283 mL | 3.4567 mL | |
| 10 mM | 0.1728 mL | 0.8642 mL | 1.7283 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04931108 | Recruiting | Drug: Dexamethasone Drug: Olanzapine Other: Placebo |
Breast Carcinoma | University of Rochester NCORP Research Base |
May 19, 2018 | Phase 3 |
| NCT03563248 | Active Recruiting |
Drug: FOLFIRINOX Drug: Losartan |
Pancreatic Cancer | Massachusetts General Hospital | August 10, 2018 | Phase 2 |
| NCT04817189 | Recruiting | Drug: NEPA (300mg netupitant/0.5mg palonosetron) Drug: Dexamethasone, 8 mg (oral) or equivalent IV dose |
Chemotherapy-induced Nausea and Vomiting |
Helsinn Healthcare SA | February 1, 2021 | Phase 4 |
| NCT06102447 | Not yet recruiting | Drug: Netopitam Palonosetron capsules and dexamethasone |
Head and Neck Squamous Cell Carcinoma (HNSCC) |
Sichuan Cancer Hospital and Research Institute |
November 1, 2023 | Not Applicable |
| NCT03204279 | Completed | Drug: Netupitant Drug: Palonosetron |
Chemotherapy-induced Nausea and Vomiting (CINV) |
Helsinn Healthcare SA | August 31, 2017 | Phase 2 |
|
|
|