| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| Other Sizes |
|
| 靶点 |
HSV-1 (IC50 = 0.02 μM); HSV-2 (IC50 = 0.02 μM)
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:Pritelivir(以前称为 AIC316,BAY 57-1293)是一种新型有效的解旋酶引物酶抑制剂,对单纯疱疹病毒(HSV)具有抗病毒作用,对 HSV-1 和 HSV-2 的 IC50 均为 20 nM。它代表了一类新的 HSV 强效抑制剂,针对病毒解旋酶引物酶复合物。 BAY 57-1293 通过抑制解旋酶引物酶并影响病毒 DNA 合成而表现出抗疱疹活性。在体外病毒复制测定中,BAY 57-1293 显示出对 HSV-1 F、HSV-2 G 和阿昔洛韦耐药 HSV-1 F 突变体的抑制作用,IC50 值为 20nM。因此,BAY 57-1293 具有治疗人类 HSV 疾病的巨大潜力,包括那些对现有药物耐药的人。作用机制是 BAY 57-1293 以剂量依赖性方式直接抑制病毒解旋酶-引物酶复合物的 ATPase 活性。 BAY 57-1293 还显示出针对阿昔洛韦耐药 HSV 突变体的有效抗病毒活性。 BAY 57-1293 减少 vero 细胞中 1 型单纯疱疹病毒诱导的 Aβ 和 P-tau。激酶测定:Pritelivir(以前称为 AIC316,BAY 57-1293)是一种新型有效的解旋酶引物酶抑制剂,对单纯疱疹病毒 (HSV) 显示出抗病毒作用,对于 HSV-1 和 HSV-2 的 IC50 均为 20 nM。细胞测定:在体外病毒复制测定中,BAY 57-1293 显示出对 HSV-1 F、HSV-2 G 和阿昔洛韦耐药 HSV-1 F 突变体的抑制作用,IC50 值为 20nM。在空斑减少测定和常规细胞致病性测定中,BAY 57-1293 显示的 IC50 值分别为 0.01-0.02μM 和 0.01-0.03μM。
|
| 体内研究 (In Vivo) |
Pritelivir 是同类中第一种针对病毒解旋酶-引物酶复合物以防止 HSV 复制的抗病毒药物[2]。普替利韦的剂量(0.03-45 mg/kg)可显着提高生存率。对于 HSV-1、E-377,普替利韦(0.3-30 mg/kg)可降低死亡率。 Pritelivir 具有强大的抗病毒活性,可以打破耐药性,使其成为治疗潜在致命的 1 型和 2 型 HSV 感染(例如单纯疱疹脑炎)的有希望的治疗方法[3]。与媒介物治疗组相比,使用 0.1 或 0.3 mg/kg/剂的 Pritelivir 和阿昔洛韦(10 mg/kg/剂)的联合治疗可预防 HSV-2、MS 株[3]。
16.6%的受试者在安慰剂组中检测到HSV脱落;在每天服用5mg的受试者中,18.2%的天数检测到Pritelivir受体脱落,在每天服用25mg的受试器中,9.3%的天数发现Pritelivir受体脱落。与安慰剂相比,Pritelivir每日剂量为5mg时病毒脱落的相对风险为1.11(95%置信区间[CI],0.65至1.87),每日剂量为25mg时为0.57(95%CI,0.31至1.03),每日用量为75mg时为0.13(95%CI:0.04至0.38),每周剂量为400mg时为0.32(95%CI,0.17至0.59)。生殖器损伤的天数百分比也显著降低,从安慰剂组的9.0%降至每天服用75mgPritelivir组的1.2%(相对风险,0.13;95%CI,0.02至0.70)和每周服用400mg的组的1.2%。所有组的不良事件发生率相似。 结论:Pritelivir以剂量依赖的方式降低了患有生殖器疱疹的健康男性和女性生殖器HSV脱落率和病变天数。(ClinicalTrials.gov编号,NCT01047540。)。[2] Pritelivir是一种解旋酶引物酶抑制剂,对人类单纯疱疹病毒(HSV)具有优异的体外和体内活性。感染HSV 1型或2型(包括阿昔洛韦耐药株)致死的小鼠在感染后72小时用Pritelivir/普立替韦或阿昔洛韦治疗7天。两种药物均单独或联合口服,每天两次。0.3至30mg/kg的Pritelivir剂量降低了HSV-1、E-377的死亡率(P<0.001)。对于阿昔洛韦耐药的HSV-1,11360,1和3 mg/kg的Pritelivir/普立韦可提高存活率(P<0.005)。对于HSV-2、MS感染的小鼠,所有高于0.3mg/kg剂量的普里特利韦均有效(P<0.005)。对于对阿昔洛韦耐药的HSV-2菌株12247,1-3mg/kg的Pritelivir剂量显著提高了存活率(P<0.0001)。与载体治疗组相比,普立韦0.1或0.3 mg/kg/剂与阿昔洛韦(10 mg/kg/剂)的联合治疗对HSV-2 MS株具有保护作用(P<0.0001)(与之前使用HSV-1的数据一致)。还观察到平均死亡天数增加(P<0.05),这表明存在潜在的协同作用。进行了药代动力学研究以确定Pritelivir浓度,发现血浆和脑样本中存在剂量依赖关系,无论感染状态或给药开始时间如何。总之,当治疗延迟到病毒接种后72小时时,普立韦被证明是有效的,并且在该模型中与阿昔洛韦联合使用似乎能协同抑制死亡率。我们得出结论,普立病毒具有强大的抗药性,有可能治疗潜在危及生命的HSV 1型和2型感染,包括单纯疱疹病毒脑炎[3]。 |
| 细胞实验 |
使用的HSV-1菌株是实验室菌株E-377和临床分离株11360,HSV-2菌株是实验室毒株MS和临床分离物12247。隔离物是杰克·希尔和巴勒斯·韦尔科姆的礼物。这些病毒的易感性之前已有报道(Prichard等人,2009;Tardif等人,2014)。菌株11360和菌株12247的ACV体外半最大有效浓度(EC50)分别大于100µM和100µM,这两种菌株都被认为对ACV具有耐药性。这些菌株具有以下多态性,并强调了ACV抗性介导的突变:菌株11360的TK多态性C6G、N23S、K36E、S181N、A192V、G251C、A265T、V267L、P268T、D286E和N376H(相对于NC_001806),菌株11360和G39E、N78D、L140F和C337Y(相对于NP044492.1),菌株12247的DNA聚合酶多态性A9T、P15S和L60P(相对于NP 044500.1),以及菌株11337的S33G、V905M、A1203T和T1208A(NC_00180.6)60。从新鲜获得的新生人类包皮制备人包皮成纤维细胞(HFF)作为原代培养物,并通过之前报道的方法在HFF细胞中制备和定量病毒储备,以供体内使用(Prichard等人,2013)。[3]
|
| 动物实验 |
Animal/Disease Models: Female balb/c (Bagg ALBino) mouse[3]
Doses: 0.03 to 45 mg/kg Route of Administration: Administered orally, twice (two times) daily at approximately 12 h intervals, for 7 days Experimental Results: Survival was Dramatically increased to 80-100% as compared to the vehicle treatment. Even the lowest dose of 0.3 mg/kg was effective in increasing survival to 53%. Pritelivir (PTV) was suspended in 1.0% carboxymethylcellulose (CMC) in water for oral delivery to mice. ACV was also suspended in 1.0% CMC for oral administration. Compounds were prepared in a 0.2 ml volume. Treatments for efficacy and drug distribution evaluations were administered to mice for 7 consecutive days beginning 24–72 h post viral inoculation by oral gavage using doses ranging from 0.03 to 45 mg/kg of Pritelivir (PTV) or 1 to 50 mg/kg of ACV given twice daily at approximately 12 h intervals depending on the specific protocol. For combination studies, data was used from previous studies which indicated that the lowest effective dose of ACV was 30 mg/kg administered orally twice daily (Prichard et al., 2011). The lowest effective dose of Pritelivir (PTV) was determined in these studies to be 0.3 mg/kg when given orally twice daily. Dose levels of 10 mg/kg for ACV and 0.3 mg/kg for Pritelivir (PTV) were selected as the highest dosages to enable the detection of improved efficacy of the combination by employing an experimental design previously reported (Quenelle et al., 2007). All mice in combination studies were dosed twice daily beginning 72 h following viral inoculation using a total volume of 0.2 ml solution of vehicle or drug solutions to equilibrate stress.[3] For pharmacokinetic studies, five mice each from vehicle and drug-treated groups were euthanized on day 8, 9 or 10 post viral inoculation following the last treatment for aseptic collection of brain and serum. Treatments were initiated at 24, 48 or 72 h post viral inoculation and uninfected mice were included for comparison. Mice were anesthetized with ketamine-xylazine via intraperitoneal injection for retro-orbital collection of whole blood using capillary tubes into a heparinized tube setting in an ice bath. Blood was stored on ice then centrifuged 134 × g for 7 min. Plasma was collected and transferred to a new vial and immediately frozen at −80°C until assayed for Pritelivir (PTV) levels by high-performance liquid chromatography coupled with mass spectrometry (HPLC-MS) (below). Mice were euthanized via carbon dioxide-oxygen asphyxiation for aseptic retrieval of brain samples. Samples were snap frozen on ethanol-dry ice and stored frozen at −80° C until assayed for Pritelivir (PTV) concentrations by HPLC-MS (below).[3] The four dosing regimens for Pritelivir were a loading dose of 20 mg followed by a daily dose of 5 mg, a loading dose of 100 mg followed by a daily dose of 25 mg, a loading dose of 300 mg followed by a daily dose of 75 mg, and a weekly dose of 400 mg. The regimen for the administration of placebo was the same as it was for the administration of pritelivir. The study drug was taken for a period of 28 days. [2] |
| 药代性质 (ADME/PK) |
Pharmacokinetic analysis of drug concentrations showed dose dependent amounts of PTV in infected and uninfected mice. Twice daily doses of 5 mg/kg, 15 mg/kg or 45 mg/kg resulted in mean plasma concentrations of 2 µg/ml, 4 µg/ml and 9 µg/ml, respectively, independent of infection status or time of initiation of dosing (Figure 5). Similarly, brain samples showed mean concentrations of approximately 0.05 µg/g for the 5 mg/kg dose group, 0.11 µg/g for the 15 mg/kg dose group and 0.22 µg/g for the mice given 45 mg/kg dose group independent of infection status or time of initiation of dosing (Figure 6). [3]
Pharmacokinetic Results [4] Single Ascending Dose (Trial 1) [4] Pritelivir plasma concentration–time profiles were characterized by a rapid initial absorption, with no apparent lag time. Maximum or near maximum plasma concentrations were reached around 1.5 hours after dosing with a plateau phase until ≈4.5 hours after dosing. This plateau phase was characterized by the occurrence of multiple absorption peaks. After the plateau phase, a multiexponential decline was observed, with a steep drop in plasma concentration (from 4.5 hours after dosing) followed by a slower decline (from 5 to 5.5 hours after dosing onward). In most subjects, a modest increase in pritelivir plasma concentration was observed between 8 and 16 hours after dosing (Figure 1A). Concentrations in the terminal phase generally declined in parallel for all dose levels (Figure 1B). The shape of pritelivir plasma concentration–time profiles in female subjects could be described similarly, although initially higher plasma concentrations were observed compared to male subjects (Figure 2A and B). PK parameters for pritelivir after single-dose administration are summarized in Table S2 for all dose groups. Cmax, AUC0-24h, AUC0-last, and AUC0-inf increased with increasing dose in the 5- to 480-mg dose range. AUC0-last and AUC0-inf could not be determined accurately for the 5- and 10-mg doses as the initial PK sampling scheme (120 hours) was not sufficient to characterize the terminal elimination phase. No further exposure increase was observed from 480 to 600 mg (Table S2, Figure 3). For the 20-to 480-mg dose range, no statistically significant deviation from dose proportionality was observed for Cmax, AUC0-24h, AUC0-last, and AUC0-inf (Table S3). The intersubject variability (%CV) was comparable between the different dose groups, ranging between 10% and 51% for Cmax and 15% and 32% for AUC0-inf. Concentration-time profiles from 20 mg onward allowed elimination half-lives to be determined accurately. Mean t1/2z ranged between 52 and 83 hours. The percentage of dose excreted in urine unchanged was negligible, not exceeding 0.3% in all dose groups. Pritelivir mean Cmax and AUC0-inf were 1.5-fold and 1.1-fold higher in females compared to males, respectively (Table S4). The intersubject variability (%CV) for Cmax was higher in women (33%) compared to men (10%) and similar for AUC0-inf (16% and 17% for men and women, respectively). Food Effect (Trial 2) [4] Mean pritelivir plasma concentrations increased more rapidly under fasted conditions compared to fed conditions, with lower mean maximum plasma concentrations. The plateau from ≈1.5 to 4.5 hours after dosing that was observed under fasted conditions was not observed under fed conditions (Figure 4). Median tmax was later under fed conditions compared to fasted conditions (5 hours compared to 3.5 hours). Mean Cmax and AUC0-last were higher under fed conditions compared to fasted conditions (Table S5), with LS means ratios of 133% and 116%, respectively (Table S6). The differences between fed and fasted conditions were statistically significant, and the upper limit of the 90%CIs of the LS means ratios were above the 125% equivalence limit. No lag time was observed under either fed and fasted conditions. Tmax was increased by 1.5 hours under fed compared to fasted conditions; however, this difference was not statistically significant. Intersubject variability (%CV) in exposure (Cmax, AUC0-last, and AUC0-inf was lower under fed conditions (13%-18%) compared to fasted conditions (22%-28%). Multiple Ascending Dose (Trials 3 and 5) [4] On both day 1 and day 21 (day 16 for the 400-mg dose group), mean pritelivir plasma concentrations increased with increasing dose (Figure 5). The shape of the pritelivir plasma concentration–time profiles was similar after single dosing (day 1) and after multiple dosing (day 21/16). Concentrations in the terminal phase generally declined in parallel for all dose levels. PK parameters for pritelivir after single-dose and multiple-dose administration are summarized in Table S7 for all dose groups. Cmax and AUC0-24h increased dose proportionally on both day 1 and day 21. Mean Cmin appeared to increase slightly less than dose proportionally from 100 to 200 mg. Note that Cmin, Cmax, and AUC0-24h for the 400-mg dose group were less accurate, as sampling started only 3 hours after dosing on day 16. Steady-state conditions were generally reached between days 8 and 13. Accumulation for Cmax and AUC0-24h ranged from 4.4-fold to 5.0-fold and from 4.4 to 5.1-fold, respectively (Table S6), based on the LS means ratios. Statistically, no significant deviation from dose proportionality across the 5- to 400-mg dose range could be shown for Cmax and AUC0-24h, on both day 1 and day 21/16, while a significant deviation from dose proportionality was observed for Cmin across the 5- to 400-mg dose range (Table S3). After pairwise comparison between the dose groups, a statistically significant difference was observed between the 100- and 200-mg dose group for Cmax on day 21 and between the 100- and 200-mg dose group and 100- and 400-mg dose group on day 21 for AUC0-24h. For Cmin, pairwise comparison showed statistically significant differences for the 5-, 25-, and 100-mg dose groups versus the 200- and 400-mg dose groups. Absolute Bioavailability (Trial 4) [4] Mean dose-normalized 14C-labeled pritelivir plasma concentrations after IV administration of a microdose were higher compared to unlabeled pritelivir plasma concentrations after oral administration (Figure 6A). The shape of the plasma concentration–time profiles was similar for 14C-labeled and unlabeled pritelivir, except at the beginning of the curve due to the lack of an absorption phase for IV-administered 14C-labeled pritelivir. Concentrations in the terminal phase declined in parallel (Figure 6B). Mean t1/2z values of 14C-labeled pritelivir were comparable to mean t1/2z values of unlabeled pritelivir. Systemic clearance and volume of distribution after IV dosing was consistent with the observed mean CL/F after oral dosing when correcting for bioavailability. Based on the mean bioavailability, absolute oral bioavailability of pritelivir was 73% (Table S8), while based on the statistical analysis of AUClast and AUC0-inf, the absolute oral bioavailability was 72%. The LS means ratios for oral unlabeled pritelivir and IV dose-normalized 14C-labeled pritelivir were 72.4% and 71.9% for AUC0-last and AUC0-inf, respectively (Table S6). |
| 毒性/毒理 (Toxicokinetics/TK) |
Safety and Tolerability [4]
For trials 1, 2, 3, 4, and 5 (up to 200-mg dose group), the administration of pritelivir was generally well tolerated at single doses ranging from 5 to 600 mg (5-600 mg for trial 1, 80 mg [fasted and fed] for trial 2, and 100 mg of unlabeled pritelivir combined with 3 μg of 14C-labeled pritelivir for trial 4) and multiple doses ranging from 5 to 200 mg once daily for 21 days for trials 3 and 5. The maximum tolerated dose was not reached with the highest dose for trials 1 and 3. There were no clinically remarkable findings or dose-related trends with respect to the safety parameters of AEs, vital signs, ECGs, or routine clinical laboratory tests. Many AEs reported were nonspecific and usually observed in clinical trials with healthy volunteers. The number of subjects with AEs was not clinically remarkably different between placebo- and pritelivir-exposed subjects (66.7% and 56.9%, respectively, for trial 1; 85.0 and 79.2%, respectively, for trial 03; and 87.5% and 79.2%, respectively, for trial 5). Neither death nor serious or other significant AEs occurred. An overview of all treatment-related AEs is presented in Table S9 (trial 1), Table S10 (trial 2), Table S11 (trial 4), and Table S12 (trials 3 and 5). In the 400-mg dose group of trial 5, an increased number of AEs was observed in nearly all subjects (number of subjects with AEs, 100%), particularly in the SOC skin and subcutaneous tissue disorders. Three subjects discontinued treatment due to AEs before day 16. Due to the AEs in these 3 subjects and adverse events in 4 additional subjects in this dose group (Tables S12 and S13), the trial was prematurely terminated in the morning of day 16 after intake of the (last) trial medication. One of the subjects (400 mg) with general physical health deterioration most likely had a viral infection as diagnosed by an ear, nose, and throat specialist; however, an allergic reaction could not be ruled out. Also, placebo-treated subjects experienced TEAEs in the SOC skin and subcutaneous disorders. Findings in laboratory parameters included elevated immunoglobulin E levels in 5 subjects on 400 mg, low neutrophil and leukocyte levels, and single events of increased C-reactive protein levels. Elevated immunoglobulin E levels of clinical relevance were also observed in one subject on placebo treatment. No clinically relevant change in vital signs and ECG parameters was observed in the 400-mg dose group. Neither death nor serious or other significant AEs occurred for all dose groups. |
| 参考文献 |
|
| 其他信息 |
Pritelivir has been used in trials studying the prevention of HSV-2 and Genital Herpes.
Pritelivir is a thiazolylamide and helicase-primase enzyme inhibitor that is active against herpes simplex virus types 1 and 2 (HSV-1 and HSV-2). Pritelivir inhibits the helicase-primase complex and prevents helicase or primase catalytic cycling of viral DNA, which interferes with DNA replication and growth. This agent does not require activation by HSV thymidine kinase and has a longer plasma half-life than nucleoside analogues. Promising new inhibitors that target the viral helicase-primase complex have been reported to block replication of herpes simplex and varicella-zoster viruses, but they have no activity against human cytomegalovirus (HCMV), another herpesvirus. The HCMV helicase-primase complex (pUL105-pUL102-pUL70) is essential for viral DNA replication and could thus be a relevant antiviral target. The roles of the individual subunits composing this complex remain to be defined. By using sequence alignment of herpesviruses homologs, we identified conserved amino acids in the putative pUL105 ATP binding site and in the putative pUL70 zinc finger pattern. Mutational analysis of several of these amino acids both in pUL105 and pUL70, proved that they are crucial for viral replication. We also constructed, by homology modeling, a theoretical structure of the pUL105 N-terminal domain which indicates that the mutated conserved amino acids in this domain could be involved in ATP hydrolysis. [1] Background: Pritelivir, an inhibitor of the viral helicase-primase complex, exhibits antiviral activity in vitro and in animal models of herpes simplex virus (HSV) infection. We tested the efficacy and safety of pritelivir in otherwise healthy persons with genital HSV-2 infection. Methods: We randomly assigned 156 HSV-2-positive persons with a history of genital herpes to receive one of four doses of oral pritelivir (5, 25, or 75 mg daily, or 400 mg weekly) or placebo for 28 days. Participants obtained daily swabs from the genital area for HSV-2 testing, which was performed with a polymerase-chain-reaction assay. Participants also maintained a diary of genital signs and symptoms. The primary end point was the rate of genital HSV shedding. [2] This study shows that pritelivir, the first in a new class of antiviral agents developed for the treatment of herpes simplex infections, is effective in suppressing viral shedding and lesion development in patients with genital herpes. Further studies will be needed to assess the magnitude of clinical benefit in the treatment of HSV infections and to establish the usefulness of pritelivir in treating severe HSV disease and in reducing sexual transmission. In May 2013, the clinical development of pritelivir was placed on hold by the Food and Drug Administration because of unexplained dermal and hematologic findings in a toxicology study of monkeys treated with daily doses ranging from 75 mg per kilogram of body weight to 1000 mg per kilogram (these doses were 70 to more than 900 times as high as a dose of 75 mg in humans). The reason for the findings in monkeys is currently under investigation; such findings were not observed in the current trial. [2] In our study, PTV demonstrated potent antiviral activity in vivo against both HSV-1 and HSV-2, including ACV resistant strains, when treatment was delayed 72 h post viral inoculation exceeding the time of treatment initiation (6 h post viral inoculation) that was reported previously (Betz et al., 2002). The delayed onset of treatment of 72 h post infection is considered to closer mimic the clinical situation where patients first develop symptoms and see the doctor before getting treatment. The activity of PTV in the combination study with ACV indicates that there is at least an additive if not a synergistic effect against HSV-2, strain MS. We previously reported potential synergy against HSV-1, E-377 in a similar study where ACV doses of 10 mg/kg combined with PTV doses of 0.3 mg/kg increased survival significantly over either agent given independently (Quenelle et al., 2016). Though further studies are needed to formally prove a synergistic effect the data demonstrate that PTV alone or in combination with ACV might be a new treatment option for HSV encephalitis as ACV is less than optimal and more effective therapy is warranted. In addition, the distinct molecular targets of the compounds (HSV helicase-primase complex for PTV and viral polymerase for ACV) would greatly reduce the risk of development of drug resistance. Furthermore, as demonstrated in our model, PTV therapy due to the different mode of action would allow for treatment of ACV-resistant infections, which also have been reported for HSV encephalitis (Kakiuchi, et. al., 2012; Schepers, et.al, 2014; Bergmann et al., 2017). It is also of importance that we could demonstrate the presence of PTV in both plasma and brain. PTV was detected in the brain of both uninfected and infected animals and the brain exposure was proportional to the exposure in plasma. As HSV encephalitis is caused by viral replication in the brain leading to acute inflammation, congestion, and/or hemorrhage (Whitley 2006), drug delivery and activity within the CNS is vital for the treatment of this condition. In the previous study (Betz et al., 2002), it was shown that viral load could be significantly reduced in the brain cortex under PTV treatment vs. parallel placebo and valacyclovir treatment. Therefore, PTV exposure in the brain is considered to be sufficient for efficacy. In human dose finding clinical trials for patients with genital HSV-2 infections, PTV reduced lesions and viral shedding. In addition, no resistant isolates of HSV-2 were found after 4 weeks of daily therapy (Edlefsen et al., 2016). These results and the new data presented here indicate that PTV might be useful for the treatment of HSV encephalitis in the future.[3] The pharmacokinetics and safety of the novel herpes simplex virus helicase-primase inhibitor pritelivir were evaluated in 5 phase 1 trials: a single-ascending-dose trial, 2 multiple-ascending-dose trials, a food-effect trial, and an absolute bioavailability trial in healthy male subjects. One cohort of healthy female subjects was included in the single-ascending-dose trial. Pritelivir pharmacokinetics were linear up to 480 mg following single and up to 400 mg following multiple once-daily doses. The half-life ranged from 52 to 83 hours, and steady state was reached between 8 and 13 days. Maximum plasma concentration and area under the plasma concentration-time curve from time 0 to the last quantifiable concentration were 1.5- and 1.1-fold higher in female compared to male subjects. Absolute bioavailability was 72% under fasted conditions. Following a fatty diet, pritelivir time to maximum concentration was 1.5 hour delayed and maximum plasma concentration and area under the plasma concentration-time curve from time 0 to the last quantifiable concentration were 33% and 16% higher, respectively. Pritelivir was safe and well tolerated up to 600 mg following single and up to 200 mg following multiple once-daily doses. Considering a therapeutic dose of 100 mg once-daily, pritelivir demonstrated a favorable safety and tolerability and pharmacokinetic profile in healthy subjects to support further development.[4] A wide dose range was tested from 5 to 600 mg (single dosing) and from 5 to 400 mg (once-daily dosing). Gender effect and food effect were tested at 80 mg and absolute bioavailability at 100 mg as the pharmacokinetics at these dose levels were well described, consistent with other dose levels, and were considered safe and well tolerated. Furthermore, the therapeutic dose was determined to be 100 mg once-daily based on PK/pharmacodynamic calculations and clinical outcomes in phase 2 trials, thus well within the range evaluated. All phase 1 trials were performed in healthy male subjects. As no specific toxicology data on maternal toxicity and embryo/fetotoxicity were available at the start of the first-in-human trial (SAD, trial 1), initially only male subjects were included. During the first-in-human SAD trial, results from 2 segment 2 studies (rat and rabbit) allowed the inclusion of women of childbearing potential. As a result, 1 group of female subjects was included to gain basic information about sex-specific variability in the pharmacokinetics of pritelivir. Additional sex data will be collected in future trials. Pritelivir plasma concentration–time profiles after oral dosing were characterized by an initial fast absorption, a plateau phase between 1.5 and 4.5 hours (under fasted conditions) and a modest increase in pritelivir concentrations between 8 and 16 hours after dosing. These characteristics may be related to the pH dependency of the administered pritelivir mesylate solubility (degree of ionization, 5.2), with low pH favoring solubility.12 The increases in plasma concentration observed between 8 and 16 hours after dosing may be caused by other absorption processes such as enterohepatic circulation. Pritelivir displays linear pharmacokinetics up to 480 mg after single dose and up to 400 mg after multiple once-daily dosing (highest multiple dose tested) with some fluctuations of the dose-normalized parameters between the different dose groups, which is not unusual considering the low number of subjects per dose group (n = 6). Following single administration of 600 mg pritelivir, there was no further increase in all exposure parameters, indicating a saturation of absorption in the gastrointestinal tract. The decrease in absorption was confirmed by an increase in CL/F at this dose level. After multiple dosing of pritelivir, steady-state conditions were generally reached between days 8 and 13; therefore, the PK parameters determined at day 16 for the 400-mg dose group were representative for steady state, although the exposure parameters for this dose group were regarded as approximations since the first PK sample was taken at 3 hours after dosing. Accumulation of exposure at steady state was about 5-fold. At a dose of 400 mg, the AUC0-inf in the SAD trial was similar to the AUC0-24h in the MAD trial at steady state, suggesting that the pharmacokinetics of pritelivir are time independent. Initial higher plasma concentrations were observed for female subjects compared to male subjects, and therefore mean Cmax was also higher (1.5-fold), while AUC0-inf was slightly higher (1.1-fold). Mean CL/F in male subjects was 1.1-fold higher compared to female subjects, while this was a decrease of about 10% after adjusting for individual body weight (Table S4). As these differences are minimal, and the sample size for comparison very small (n = 6 per group), it is not possible to draw a definite conclusion on the effect of sex or body weight on the pharmacokinetics of pritelivir. This will be further investigated in future studies that include a higher number of women. The effect of food was evaluated at a dose of 80 mg under fasted and fed conditions (high-fat breakfast). After a high-fat breakfast, median tmax was 1.5 hours later compared to fasted conditions due to slower initial absorption. Mean Cmax and AUC0-last were 33% and 16% higher, respectively. The clinical relevance of this increase should be interpreted with caution, as a more conventional kind of meal might affect the pharmacokinetics of pritelivir to a lesser extent. Overall, due to the high oral bioavailability and the low impact of food, pritelivir can be taken irrespective of food in future trials. The pharmacokinetics of pritelivir after an IV dose were determined in an absolute bioavailability trial. Use of the sensitive AMS technique made it possible to measure 14C-labeled pritelivir in plasma after a dose of only 3 μg (9.3 kBq), thereby minimizing radiation exposure. The IV dose was timed to coincide with tmax after 100-mg oral dosing to ensure that the pharmacokinetics of the microdose reflected those at pharmacologically relevant plasma concentrations. Oral bioavailability did not show any absorption issues and was high (72%) based on bioavailability, thus supporting oral dosing. The observed mean volume of distribution for 14C-labeled pritelivir (79 L) was substantially greater than the total volume of body water (±42 L), indicating distribution of pritelivir into tissues. The mean clearance after IV infusion (0.82 L/h) was much lower than the hepatic blood flow in humans (±87 L/h), indicating that pritelivir is a low-clearance drug. After an oral dose of 100 mg, CL/F was 1.18 L/h, reflecting the absolute bioavailability. Single oral doses of pritelivir up to 600 mg in healthy male subjects and 80 mg in female subjects were well tolerated as well as multiple doses up to 200 mg once daily for 21 days. There were no clinically remarkable findings with respect to the safety parameters, and there was no trend or dose relation with regard to TEAEs. In the placebo-controlled trials, the number of subjects with TEAEs and their pattern and incidence were not clinically remarkably different between placebo and pritelivir-exposed subjects. At 400 mg once daily, multiple subjects experienced AEs belonging to the SOC skin and subcutaneous tissue disorders (for which 2 were of moderate intensity and others of mild intensity), and therefore trial 5 was stopped prematurely. It should be noted that this occurred after pritelivir as well as after placebo dosing and that pritelivir exposures were ≈4-fold higher compared to the anticipated therapeutic dose/exposure after 100-mg once-daily dosing. Skin observations were generally mild and resolved quickly, the effects were skin bounded and self-limiting and no pustular reaction and no signs of severe systemic processes (no drug rash with eosinophilia and systemic symptoms) occurred. As several subjects had signs and symptoms of viral infection in the pritelivir and placebo group, this could have been the cause; however, an effect of pritelivir could not be completely ruled out. Pritelivir is an inhibitor of carbonic anhydrase isoenzymes.15 Approved carbonic anhydrase inhibitors such as acetazolamide have been associated with the following adverse drug reactions when used in therapeutic amounts: urinary urgency, deterioration of performance, hearing impairment, acidosis, hypercalciuria, depression, and hepatic impairment. Therefore, a potential inhibitory effect on carbonic anhydrases was evaluated by measuring the renal excretion of Na+, K+, and Cl– in trial 1. No effect on the renal excretion of sodium, potassium, and chloride was detected in the different dose groups in urine samples for all collection periods up to 48 hours after dosing, indicating that no apparent inhibition of carbonic anhydrases occurred at the doses administered. Based on the results from these trials, dosing regimens were selected for further development to yield trough concentrations suppressing viral replication over the entire dosing interval. The calculated 90% effective concentration (EC90) of 66 ng/mL for HSV-2 was used as a threshold based on the in vitro 50% effective concentration of 12 ng/mL.6 After correcting plasma concentrations from the MAD trials for plasma protein binding (2.8%, determined by equilibrium analysis; data on file), unbound steady-state trough concentrations were ≈5, 23, 105, and 157 ng/mL for the dosing regimens of 5, 25, 100, and 200 mg once daily, respectively, thus indicating that doses from 100 mg once daily onward will result in plasma concentrations that exceed the EC90 over the entire dosing interval. Based on these data, 100 mg once daily was selected for the currently ongoing phase 3 trial in immunocompromised patients with acyclovir resistant mucocutaneous HSV infection (PRIOH-1, NCT03073967) and is successfully used in an international early-access program with acyclovir-resistant and foscarnet-resistant or acyclovir-resistant and foscarnet-intolerant immunocompromised patients with HSV.16, 17 Overall, the PK characteristics of pritelivir include a high oral availability, supporting oral drug administration, a dose-dependent behavior over a large dose range, a Vd indicating extravascular tissue distribution, trough concentration levels at doses from 100 mg once daily onward exceeding the in vitro EC90 for inhibition of viral replication and safety and tolerability data supporting a dosing regimen of up to 200 mg once daily. This supports the use of pritelivir as a new HSV drug for further development with dosing regimens that would yield sufficient high concentrations for a continuous suppression of HSV replication.[4] |
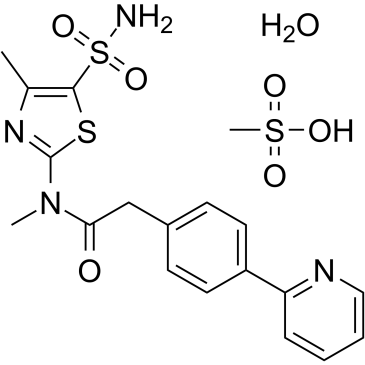
| 分子式 |
C19H24N4O7S3
|
|---|---|
| 分子量 |
516.61
|
| 精确质量 |
516.08
|
| CAS号 |
1428321-10-1
|
| 相关CAS号 |
Pritelivir;348086-71-5;Pritelivir mesylate;1428333-96-3
|
| PubChem CID |
71515453
|
| 外观&性状 |
White to light yellow solid powder
|
| tPSA |
207
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
11
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
33
|
| 分子复杂度/Complexity |
709
|
| 定义原子立体中心数目 |
0
|
| SMILES |
C1=NC(C2C=CC(=CC=2)CC(N(C)C2SC(S(N)(=O)=O)=C(N=2)C)=O)=CC=C1.S(=O)(O)(=O)C.O
|
| InChi Key |
QPIDAZSAUYNBAC-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C18H18N4O3S2.CH4O3S.H2O/c1-12-17(27(19,24)25)26-18(21-12)22(2)16(23)11-13-6-8-14(9-7-13)15-5-3-4-10-20-15;1-5(2,3)4;/h3-10H,11H2,1-2H3,(H2,19,24,25);1H3,(H,2,3,4);1H2
|
| 化学名 |
methanesulfonic acid;N-methyl-N-(4-methyl-5-sulfamoyl-1,3-thiazol-2-yl)-2-(4-pyridin-2-ylphenyl)acetamide;hydrate
|
| 别名 |
Pritelivir mesylate hydrate; 1428321-10-1; Pritelivir (mesylate hydrate); 7EX4CZ4HYG; AIC316 (mesylate hydrate); Pritelivir mesylate monohydrate; CHEMBL5194635; SCHEMBL14795919;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9357 mL | 9.6785 mL | 19.3570 mL | |
| 5 mM | 0.3871 mL | 1.9357 mL | 3.8714 mL | |
| 10 mM | 0.1936 mL | 0.9678 mL | 1.9357 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。