| 规格 | 价格 | ||
|---|---|---|---|
| 5mg | |||
| Other Sizes |
| 靶点 |
cAMP analog
|
|---|---|
| 体外研究 (In Vitro) |
8-Bromo-cAMP 钠盐是环 AMP 的溴化衍生物,可增强细胞重编程。 8-Bromo-cAMP 钠盐可提高人类新生儿包皮成纤维细胞 (HFF1) 的重编程效率。 8-Bromo-cAMPodium salt 可减少恶性胶质瘤细胞系 (A-172) 和食道癌细胞系 (Eca-109) 的增殖、分化和死亡 [3]。
|
| 体内研究 (In Vivo) |
研究了腺嘌呤核苷酸对犬缺血性心肌顿抑的影响。对戊巴比妥麻醉的开胸犬进行20分钟的左前降支冠状动脉(LAD)结扎,然后再灌注30分钟。在整个实验过程中,以0.1 ml/kg/min的速度将生理盐水、5 mM 8-溴-5'-AMP(三丁基AMP)或30 mM N6、2'、3'-三丁基-5'-AMP、5 mM 5-氨基-4-咪唑甲酰胺核糖核苷(AICAr)作为阳性对照注入左股静脉。通过超声波测量心肌收缩功能。测定再灌注心脏中高能磷酸盐的组织水平。盐水灌注组通过节段缩短百分比(%SS)评估的心肌收缩功能在缺血期间降低,在再灌注期间恢复到缺血前水平,但不完全。在输注8-溴-AMP-和AICAr的组中观察到再灌注期间SS百分比的显著改善,但在输注三丁基AMP的组中没有。再灌注期间药物对心肌收缩性的保护作用大小为8-溴-AMP>AICAr>三丁基-AMP=生理盐水。仅在8-溴-AMP输注组中,再灌注心脏中的ATP、ADP和总腺嘌呤核苷酸水平显著高于盐水输注组。目前的结果表明,8-溴-AMP提高了心脏从缺血和再灌注中恢复的能力,这与ATP的显著恢复有关[2]。
|
| 酶活实验 |
人类子宫内膜蜕膜化是一个涉及生化和形态学变化的分化过程,是胚胎植入和成功怀孕的先决条件。在这里,我们表明雷帕霉素的哺乳动物靶点(mTOR)是8-溴腺苷3',5'-环单磷酸(8-Br-cAMP)诱导的人子宫内膜基质细胞蜕膜化的关键调节因子。在8-Br-cAMP诱导的蜕膜化过程中,mTOR复合物2(mTORC2)中的mSin1和mTOR复合体1(mTORC1)中的DEPTOR水平降低,导致mTORC2活性降低,mTORC1活性升高。值得注意的是,DEPTOR置换增加了猛禽和胰岛素受体底物-1(IRS-1)之间的关联,促进了IRS-1在丝氨酸636/639的磷酸化。最后,在蜕膜化过程中,Akt的S473和T308磷酸化都降低了,随后叉头盒O1(FOXO1)磷酸化降低,蜕膜化标志物催乳素(PRL)和胰岛素样生长因子结合蛋白-1(IGFBP-1)的mRNA水平升高。综上所述,我们的研究结果揭示了mTOR在蜕膜化中的关键作用,涉及mTORC1和mTORC2的差异调节[5]。
|
| 细胞实验 |
将培养的Eca-109细胞分为四组:E1组(与8-Br-cAMP 共培养24小时);E2组(与8-Br-cAMP 共培养48h);C1组(不含8-Br-cAMP处理24小时);C2组(不含8-Br-cAMP处理48小时)。将每组相同浓度的细胞悬浮液分别滴在载玻片和硝化纤维膜(NCM)上。制备c-myc、野生型p53、bcl-2和iNOS的生物素标记cDNA探针进行原位杂交。采用免疫细胞化学方法检测表皮生长因子受体(EGFR)、p38激酶、FAS、FasL和caspase-3的表达,并用细胞化学方法检查NOS活性和分化细胞/增殖细胞的比例。对每组的载玻片和NCM标本分别进行免疫细胞化学、细胞化学和原位杂交。此外,采用TUNEL法检测各组细胞凋亡率。[4]
结果:E2组的凋亡率明显高于E1组,而E1组和E2组的分化细胞/增殖细胞比率没有差异。E1组wt p53和iNOS信号明显强于C1组,而c-myc和EGFR信号明显弱于C1组(P<0.05)。此外,E2组wt p53、iNOS、p38激酶、caspase-3和NOS活性的信号明显强于C2组,而bcl-2、c-myc和Fas/FasL的信号明显弱于C2组(P<0.05)。[4] 结论:8-Br-cAMP 可分别诱导人食管癌症Eca-109细胞经24小时和48小时分化和凋亡。wt p53、iNOS的上调和c-myc的下调可能与Eca-109细胞的分化和凋亡有关。此外,FasL、p38激酶和caspase-3的上调以及bcl-2和Fas的下调可能与Eca-109细胞的凋亡有关。[4] |
| 动物实验 |
Thirty-six mice received the implantation of CT26 carcinoma tissue in their cecum. After general anesthesia and sterilization, a 2 cm vertical incision was made at the right lower quadrant of the abdomen. The cecum was then pulled out of the abdomen. The serosa of the cecum that was exposed out was scratched, and a 2 mm diameter tumor tissue was attached with fibrin glue. Finally, the cecum was put back into place and the skin was sealed. After tumor implantation, mice were randomly divided into a control group and an experimental group. In the experimental group, the intraperitoneal injection of 8-Br-cAMP (60 mg/kg/day) was performed for 7 days, while control mice received injection of normal saline. Mice were sacrificed on the 7th, 14th, and 28th days, and tumor tissue was harvested for the evaluation of gene expression. However, due to a high mortality rate, the number of mice for sacrifice at each time point was adjusted in order to guarantee that mice were available for culling on the 28th day. [6]
|
| 参考文献 |
[1]. Jiang J, et, al. Evidences for involvement of endogenous cAMP in Arabidopsis defense responses to Verticillium toxins. Cell Res. 2005 Aug;15(8):585-92.
[2]. Nakai T, et, al. Effects of adenine nucleotide analogues on myocardial dysfunction during reperfusion after ischemia in dogs. J Cardiovasc Pharmacol. 1996 Aug;28(2):264-70. [3]. A cyclic AMP analog, 8-Br-cAMP, enhances the induction of pluripotency in human fibroblast cells. Stem Cell Rev. 2011 Jun;7(2):331-41. [4]. Dual effects of 8-Br-cAMP on differentiation and apoptosis of human esophageal cancer cell line Eca-109. World J Gastroenterol. 2005 Nov 7;11(41):6538-42. [5]. Differential regulation of mTORC1 and mTORC2 is critical for 8-Br-cAMP-induced decidualization. Exp Mol Med. 2018 Oct 30;50(10):1-11. [6]. Angiogenesis and vasculogenic mimicry are inhibited by 8-Br-cAMP through activation of the cAMP/PKA pathway in colorectal cancer. Onco Targets Ther. 2018 Jul 2;11:3765-3774. |
| 其他信息 |
Although there were reports suggesting the involvement of endogenous cAMP in plant defense signaling cascades, there is no direct evidence supporting this notion yet and the detailed mechanism is unclear. In the present study, we have used pathogenic fungi Verticillium dahliae and Arabidopsis plants as a model system of plant-microb interaction to demonstrate the function of endogenous cAMP in Arabidopsis defense responses. Both V. dahliae inoculation and Verticillium toxins injection induced typical "wilt" symptoms in Arabidopsis seedlings. When either 8-Br-AMP (a membrane permeable cAMP analogue) or salicylic acid (SA) was applied to Arabidopsis, the plants became resistant to V. dahliae toxins. However, addition of 8-Br-AMP did not increase the resistance of Arabidopsis transgenic plants deficient in SA to the toxins, suggesting that cAMP might act upstream of SA in plant defense signaling pathway. Indeed, 8-Br-cAMP and forskolin, an activator of adenylyl cyclase, significantly stimulated the endogenous SA level in plants, whereas DDA, an inhibitor of adenylyl cyclase dramatically reduced toxin-induced SA increase. Both the endogenous cAMP and SA increased significantly in Arabidopsis seedlings treated with toxins. Furthermore, transcription level of pathogenesis-related protein 1 gene (PR1) was strongly induced by both 8-Br-cAMP and the toxin treatment. Taken together, our data demonstrate that endogenous cAMP is involved in plant defense responses against Verticillium-secreted toxins by regulating the production of the known signal SA in plant defense pathway.[1]
|
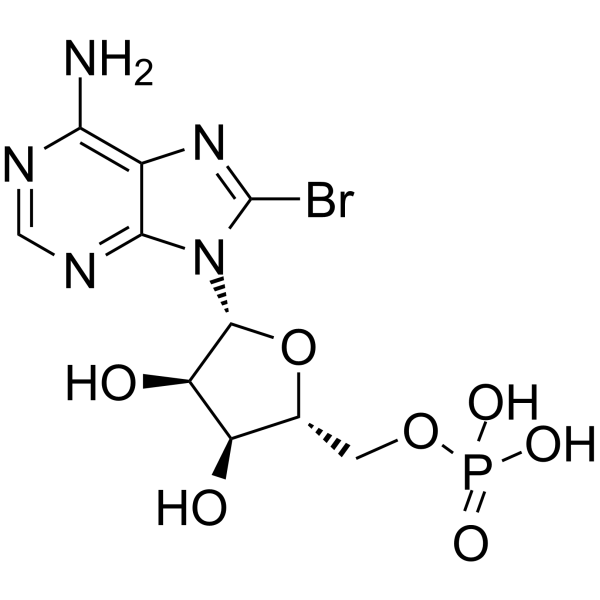
| 分子式 |
C10H13BRN5O7P
|
|---|---|
| 分子量 |
426.12
|
| 精确质量 |
422.958
|
| CAS号 |
23567-96-6
|
| 相关CAS号 |
8-Bromo-cAMP sodium salt;76939-46-3
|
| PubChem CID |
168120
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
0.357
|
| tPSA |
201.54
|
| 氢键供体(HBD)数目 |
5
|
| 氢键受体(HBA)数目 |
11
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
24
|
| 分子复杂度/Complexity |
514
|
| 定义原子立体中心数目 |
4
|
| SMILES |
C1=NC(=C2C(=N1)N(C(=N2)Br)[C@H]3[C@@H]([C@@H]([C@H](O3)COP(=O)(O)O)O)O)N
|
| InChi Key |
DNPIJKNXFSPNNY-UUOKFMHZSA-N
|
| InChi Code |
InChI=1S/C10H13BrN5O7P/c11-10-15-4-7(12)13-2-14-8(4)16(10)9-6(18)5(17)3(23-9)1-22-24(19,20)21/h2-3,5-6,9,17-18H,1H2,(H2,12,13,14)(H2,19,20,21)/t3-,5-,6-,9-/m1/s1
|
| 化学名 |
[(2R,3S,4R,5R)-5-(6-amino-8-bromopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl dihydrogen phosphate
|
| 别名 |
23567-96-6; 8-Bromo-AMP; 8-BROMO-ADENOSINE-5'-MONOPHOSPHATE; 8-Bromoadenosine 5'-monophosphate; 8-Bromoadenosine 5'-(dihydrogen phosphate); 5'-Adenylic acid, 8-bromo-; CHEMBL1230617; 8-Bromo-Adenosine Mono Phosphate;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O: 125 mg/mL (293.34 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3468 mL | 11.7338 mL | 23.4676 mL | |
| 5 mM | 0.4694 mL | 2.3468 mL | 4.6935 mL | |
| 10 mM | 0.2347 mL | 1.1734 mL | 2.3468 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。