| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50g |
|
||
| 100g |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
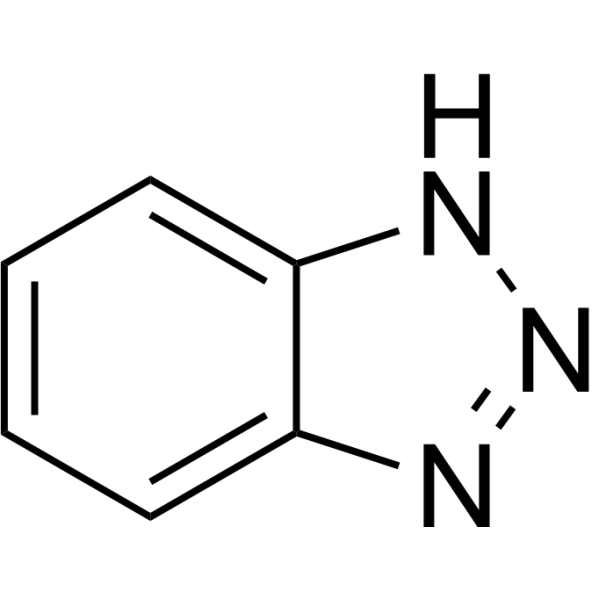
1,2,3-苯并三唑,化学式C6H5N3,是具有三个氮原子的杂环分子。这种极性、无色芳香族分子有许多应用。
|
|---|---|
| 药代性质 (ADME/PK) |
Metabolism / Metabolites
Benzotriazoles (BTs) are xenobiotic contaminants widely distributed in aquatic environments and of emerging concern due to their polarity, recalcitrance, and common use. During some water reclamation activities, such as stormwater bioretention or crop irrigation with recycled water, BTs come in contact with vegetation, presenting a potential exposure route to consumers. We discovered that BT in hydroponic systems was rapidly (approximately 1-log per day) assimilated by Arabidopsis plants and metabolized to novel BT metabolites structurally resembling tryptophan and auxin plant hormones; <1% remained as parent compound. Using LC-QTOF-MS untargeted metabolomics, we identified two major types of BT transformation products: glycosylation and incorporation into the tryptophan biosynthetic pathway. BT amino acid metabolites are structurally analogous to tryptophan and the storage forms of auxin plant hormones. Critical intermediates were synthesized (authenticated by (1)H/(13)C NMR) for product verification. In a multiple-exposure temporal mass balance, three major metabolites accounted for >60% of BT. Glycosylated BT was excreted by the plants into the hydroponic medium, a phenomenon not observed previously. The observed amino acid metabolites are likely formed when tryptophan biosynthetic enzymes substitute synthetic BT for native indolic molecules, generating potential phytohormone mimics. These results suggest that BT metabolism by plants could mask the presence of BT contamination in the environment. Furthermore, BT-derived metabolites are structurally related to plant auxin hormones and should be evaluated for undesirable biological effects. 1-H-Benzotriazole was metabolized by rat liver microsomes in vitro to 4-hydroxybenzotriazole and 5-hydroxybenzotriazole. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: 1,2,3-Benzotriazole (BT) is a white to light tan, crystalline powder. It is used as photographic restrainer and a chemical intermediate. It is also a corrosion inhibitor in industrial water treatment, and in the treatment of bronze disease in metal fine art conservation. HUMAN STUDIES: A report showed that two metal workers developed contact dermatitis from exposure to lubricating oil that contained BT. ANIMAL STUDIES: In a test for primary skin irritation and sensitization on guinea pigs, BT was at most mildly irritating in concentrations up to 50% in ethanol and was not a sensitizer. The dry powder is severely irritating to rabbit eyes (0.1 mL unwashed) but prompt water washing reduces irritation considerably. BT was positive in the Salmonella typhimurium and Escherichia coli mutagenicity assays. ECOTOXICITY STUDIES: This chemical has been widely detected in aquatic environments and shows some degree of environmental persistence. BT exposure can negatively affect endocrine systems and can result in neurotoxicity in fish. BT demonstrated hepatotoxicity and neurotoxicity in Chinese rare minnow. In female marine medaka, exposure to 0.01 mg/L BT caused notable changes in expression levels of vitellogenin, CYP1A1 and CYP19a. In vitro assays conducted using a recombinant yeast (anti-) estrogen assay indicated that BT possessed clear antiestrogenic properties. BT metabolism by plants could mask the presence of BT contamination in the environment. Furthermore, BT-derived metabolites are structurally related to plant auxin hormones. Toxicity Data LC50 (rat) = 1,910 mg/m3/3H Interactions Benzotriazole (BTR), an emerging class of environmental pollutant, is widely used in industrial applications and household dishwashing agents. Despite the reported toxicity of BTR to aquatic organisms, little is known about its effects on terrestrial invertebrates. Copper (Cu) accumulates in agricultural soils receiving urban waste products, fertilizers, fungicides, and urban sewage. In this study, two different types of bioassays (acute toxicity test and behavioral toxicity test) were performed to evaluate the toxicity of Cu and BTR, both singly and together, on the earthworm (Eisenia fetida) in artificial soil. The results of avoidance behavior tests showed that the EC50 (48 hr) values for Cu and BTR were 1.47 and 0.46 mmol/kg, respectively. The results of the acute toxicity tests showed that the LC50 (7 d) and LC50 (14 d) of Cu in earthworms were 9.19 and 5.28 mmol/kg, respectively, and the LC50 (7 d) and LC50 (14 d) of BTR were 2.43 and 1.76 mmol/kg, respectively. Toxicity analysis demonstrated that the binary BTR and Cu mixture had predominantly antagonistic effects on the avoidance behavior and survival of earthworms. The Cu2+ activities and mortality of earthworms decreased significantly with increasing concentrations of BTR, while the solid-liquid distribution coefficient of Cu increased. These results indicated that the presence of BTR can reduce the toxicity as well as the bioavailability of Cu in soil with both BTR and Cu. As an emerging contaminant, 1-H-benzotriazole (1H-BTR) has been detected in the engineered and natural aquatic environments, which usually coexists with heavy metals and causes combined pollution. In the present study, wild-type and transgenic zebrafish Danio rerio were used to explore the acute toxicity as well as the single and joint hepatotoxicity of cadmium (Cd) and 1H-BTR. Although the acute toxicity of 1H-BTR to zebrafish was low, increased expression of liver-specific fatty acid binding protein was observed in transgenic zebrafish when the embryos were exposed to 5.0 uM of 1H-BTR for 30 days. Besides, co-exposure to 1H-BTR not only reduced the acute toxic effects induced by Cd, but also alleviated the Cd-induced liver atrophy in transgenic fish. Correspondingly, effects of combined exposure to 1H-BTR on the Cd-induced expressions of several signal pathway-related genes and superoxide dismutase and glutathione-s-transferase proteins were studied. Based on the determination of Cd bioaccumulation in fish and the complexing stability constant (beta) of Cd-BTR complex in solution, the detoxification mechanism of co-existing 1H-BTR on Cd to the zebrafish was discussed. Non-Human Toxicity Values LC50 Rat inhalation 1900 mg/cu m/3 hr LD50 Rat oral 600 mg/kg LD50 Mouse oral 615 mg/kg LD50 Mouse ip 400 mg/kg For more Non-Human Toxicity Values (Complete) data for 1,2,3-Benzotriazole (6 total), please visit the HSDB record page. |
| 其他信息 |
1,2,3-benzotriazole appears as white to light tan crystals or white powder. No odor. (NTP, 1992)
Benzotriazole is the simplest member of the class of benzotriazoles that consists of a benzene nucleus fused to a 1H-1,2,3-triazole ring. It has a role as an environmental contaminant and a xenobiotic. |
| 分子式 |
C6H5N3
|
|---|---|
| 分子量 |
119.12
|
| 精确质量 |
119.048
|
| CAS号 |
95-14-7
|
| 相关CAS号 |
1H-Benzotriazole-4,5,6,7-d4;1185072-03-0
|
| PubChem CID |
7220
|
| 外观&性状 |
Needles from chloroform or benzene
White to light tan, crystalline powder |
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
204 ºC (15 mmHg)
|
| 熔点 |
97-99 °C(lit.)
|
| 闪点 |
170 ºC
|
| 蒸汽压 |
0.0±0.8 mmHg at 25°C
|
| 折射率 |
1.715
|
| LogP |
1.34
|
| tPSA |
41.57
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
0
|
| 重原子数目 |
9
|
| 分子复杂度/Complexity |
92.5
|
| 定义原子立体中心数目 |
0
|
| SMILES |
N1NC2C(=CC=CC=2)N=1
|
| InChi Key |
QRUDEWIWKLJBPS-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C6H5N3/c1-2-4-6-5(3-1)7-9-8-6/h1-4H,(H,7,8,9)
|
| 化学名 |
2H-benzotriazole
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 8.3949 mL | 41.9745 mL | 83.9490 mL | |
| 5 mM | 1.6790 mL | 8.3949 mL | 16.7898 mL | |
| 10 mM | 0.8395 mL | 4.1974 mL | 8.3949 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。