| 规格 | 价格 | ||
|---|---|---|---|
| 500mg | |||
| 1g | |||
| Other Sizes |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
/MILK/ Nonnutritive sweeteners (NNS), including saccharin, sucralose, aspartame, and acesulfame-potassium, are commonly consumed in the general population, and all except for saccharin are considered safe for use during pregnancy and lactation. Sucralose (Splenda) currently holds the majority of the NNS market share and is often combined with acesulfame-potassium in a wide variety of foods and beverages. To date, saccharin is the only NNS reported to be found in human breast milk after maternal consumption, while there is no apparent information on the other NNS. Breast milk samples were collected from 20 lactating volunteers, irrespective of their habitual NNS intake. Saccharin, sucralose, and acesulfame-potassium were present in 65% of participants' milk samples, whereas aspartame was not detected. These data indicate that NNS are frequently ingested by nursing infants, and thus prospective clinical studies are necessary to determine whether early NNS exposure via breast milk may have clinical implications. /Acesulfame potassium/ Single oral doses of 10 mg (14)C-Acesulfame K/kg bw given to rats and dogs were rapidly absorbed. Maximum blood levels reached were 0.75 plus or minus 0.2 g/mL in rats, 0.5 hr after dosing, and 6.56 plus or minus 2.08 g/mL in dogs, 1-1.5 hr after dosing. In rats, 82-100% of the dose, and in dogs, 85-100% of the dose was excreted in the urine; in both species, 97-100% of the total radioactivity was excreted in feces, and total recovery approximated 100%. Rats given 10 consecutive daily doses of 10 mg/kg orally did not show evidence of accumulation. Three days after dosing, the concn in the organs and plasma was 0.4 nMol/g in liver, and <0.2 nMol/g in other tissues. Seven days after dosing, the concn in dogs was <0.2 nMol/g in all tissues examined. /Acesulfame K/ After pretreatment for seven days with a diet containing 3% Acesulfame K, male rats were given a dose of 250 mg Acesulfame K containing (14)C-Acesulfame K (9.6 x 108 dpm) by oral gavage. After eight hours the animals were killed and liver and spleen excised; DNA and chromatin protein was isolated from these organs. No radioactivity could be detected on any DNA sample. A low level of activity (8-11 dpm/mg protein) was associated with chromatin protein and this was claimed to be due to non-covalent interactions of unchanged Acesulfame K. /Acesulfame K/ Single oral doses of approximately 15 mg (14)C-Acesulfame K/kg bw were administered to male and female rats which had been pretreated with unlabeled Acesulfame K at a level of 300 mg/kg diet for 60 days. Control animals without pretreatment were also similarly dosed with (14)C-Acesulfame K. In all animals 95.1-98.2% of the dose was recovered in urine and cage washings and 0.95-2.86% in feces. Total recoveries were 96.3-99.2%. Excretion of radioactivity was rapid and displayed biphasic kinetics; 92.6-96.8% of the dose was excreted in 24 hours. ... No significant differences in route or rate of excretion were observed between sexes nor between controls and animals pretreated with Acesulfame K for 60 days. /Acesulfame K/ For more Absorption, Distribution and Excretion (Complete) data for Acesulfame (8 total), please visit the HSDB record page. Metabolism / Metabolites The metabolism of Acesulfame K was investigated in the urine and feces of rats and dogs which had received single oral doses of 10 mg/kg bw, and in the urine and bile of pigs dosed orally with 5 mg/kg bw. The analytical methods used (thin-layer chromatography, mass spectrometry and isotope dilution) detected only the original substance in these samples. Separation by TLC of urinary extracts from rats used in the above study revealed only one peak which was identical with Acesulfame K. No metabolites were detected in control or Acesulfame K-pretreated animals. Similarly, no metabolites were detected in animals which had been pretreated with 1% Acesulfame K for 7 days. /Acesulfame K/ The metabolism of Acesulfame K was studied in serum and urine from human volunteers following a single dose of 30 mg/individual. Only the original substance was detected in all samples. /Acesulfame K/ Biological Half-Life /After/ single oral doses of approximately 15 mg (14)C-Acesulfame K/kg bw were administered to male and female rats which had been pretreated with unlabeled Acesulfame K at a level of 300 mg/kg diet for 60 days; ... the half-life of the rapid phase was 4-4.5 hours and of the slower phase (accounting for <0.5% of the dose) was 109-257 hours. /Acesulfame K/ After iv admin of a single dose of 10 mg (14C)-Acesulfame K/kg bw to rats, the radioactivity was excreted quantitatively in urine and the plasma half-life was 0.23 hr. /Acesulfame K/ In lactating rats given a single oral dose of (14)C-Acesulfame K of about 10.6 mg/kg bw, ...the biological half-lives were similar in milk (5.6 hr) and blood (4 hr). /Acesulfame K/ The purpose of this work was...the determination of acesulfame-K (AcK) in C57BL mouse plasma and urine. Male and female animals were dosed orally (gavage) and intravenously at 10 mg/kg and then plasma and urine collected at up to 24 hours post dose. Plasma samples were analyzed with an HPLC method... and urine was analyzed with a second HPLC method .... Both methods used saccharin as internal standard with detection by UV absorbance at 230 nm. Following IV administration, plasma AcK concentrations declined rapidly and linearly within 120 min and a second AcK peak was observed at 240 minutes. Half-life was estimated to be 11-15 minutes. Plasma concentrations of AcK reached maximal levels within 45 minutes and rapidly declined following oral doses. Plasma AcK were below limits of detection by 480 minutes post dose. A second peak was also observed following oral administration, suggesting enterohepatic recirculation. Following IV and PO administration, 45% (males) and 70% (females) of the dose was excreted in urine by 24 hr. Oral bioavailability was estimated to be 90-100% based on urinary data. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Acesulfame (AS) is a solid. It is used as artificial sweetener for food, also used in cosmetics. HUMAN STUDIES: A case study is reported whereby an individual with known sulfite and sulfonamide allergies develops hypersensitivity to taurine above a threshold level as well as to the non-nutritive sweetener acesulfame potassium, compounds that are not normally associated with allergic reactions. In vitro studies suggested that long-term consumption of AS might accelerate atherosclerosis and senescence via impairment of function and structure of apoA-I and HDL. ANIMAL STUDIES: Acesulfame K was non-irritant in a primary dermal irritation test in the rabbit. Acesulfame K showed no antigenic effect and only the guinea pigs sensitized with BSA showed anaphylactic reactions. Acesulfame K was not carcinogenic in mice or rats. A multigeneration study in rats was carried out, in which males and females received Acesulfame K at dietary levels of 0, 0.3, 1.0 and 3.0% for three successive generations, each comprising two consecutive litters. Growth rate was slightly decreased in the top dose group of the F0 and F1 generations, and the mid-dose group of the F0 generation. In the teratogenicity studies, no adverse effects were seen in appearance, food consumption, autopsy of the dams, organ weights, or litter data; no visceral or skeletal abnormalities attributable to the treatment were observed. Acesulfame K was negative in the genotoxicity studies in vivo and in vitro, including Ames tests in S. typhimurium TA98, TA100, TA15325, TA1537, TA1538 at 0-100 mg/plate, 4-5000 ug/plate and in E.Coli WP2uvrA at 4-5000 ug/plate. ECOTOXICITY STUDIES: AS is listed as an emerging contaminant due to its environmental persistence and wide occurrence in the environment. An increased toxicity of AS after UV irradiance was observed in the liver of Carassius auratus exposed to AS and its irradiation products. Embryotoxicity tests showed that AS transformation products at the low g/L level produced significant adverse effects in tail detachment, heart rate, hatching rate and survival rate during fish embryo development. Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Acesulfame has been found in variable concentrations in the breastmilk of nursing mothers who report consuming artificially sweetened beverages and sweetener packets in the past 24 hours. Even some mothers who reported not consuming artificial sweeteners have small amounts of acesulfame in their breastmilk. However, it is not likely to reach an intake greater than the acceptable daily intake for infants. Ingestion of diet drinks containing low-calorie sweeteners might increase the risk of vomiting in breastfed infants. Some authors suggest that women may wish to limit the consumption of nonnutritive sweeteners while breastfeeding because their effect on the nursing infants are unknown. ◉ Effects in Breastfed Infants A cross-sectional survey assessed the dietary history of US mothers nursing infants between 11 and 15 weeks of age. The survey was used to estimate the amount of diet soda and fruit drinks consumed by the women. There were no statistically significant differences in infants’ weight or z-scores based on low calorie sweetener exposure. However, infants exposed to low calorie sweetener in milk once or less per week had a statistically significantly higher risk of vomiting than those who were not exposed. Greater exposure was not associated with vomiting. It was not possible to assess the effects of specific sweeteners. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Interactions The ecotoxicity of heavy metals depends much on their speciation, which is influenced by other co-existing substances having chelating capacity. In the present study, the toxic effects of Cd(2+) and Cu(2+) on a green algae Scenedesmus obliquus were examined in the presence of two artificial sweeteners (ASs), acesulfame (ACE) and sucralose (SUC) by comparing the cell specific growth rate mu and pulse-amplitude-modulated (PAM) parameters (maximal photosystem II photochemical efficiency Fv/Fm, actual photochemical efficiency Yield, and non-photochemical quenching NPQ) of the algae over a 96-hr period. Simultaneously, the bioconcentration of the metals by the algal cells in the presence of the ASs was measured. The presence of ACE enhanced the growth of S. obliquus and promoted the bioconcentration of Cd(2+) in S. obliquus, while the impacts of SUC were not significant. Meanwhile, EC50 values of Cd(2+) on the growth of S. obliquus increased from 0.42 mg/L to 0.54 mg/L and 0.48 mg/L with the addition of 1.0 mg/L ACE and SUC, respectively. As for Cu(2+), EC50 values increased from 0.13 mg/L to 0.17 mg/L and 0.15 mg/L with the addition of 1.0 mg/L ACE and SUC, respectively. In summary, the two ASs reduced the toxicity of the metals on the algae, with ACE showing greater effect than SUC. Although not as sensitive as the cell specific growth rate, PAM parameters could disclose the mechanisms involved in metal toxicity at subcellular levels. This study provides the first evidence for the possible impact of ASs on the ecotoxicity of heavy metals. Swiss Albino male mice were exposed to blends of aspartame (3.5, 35, 350 mg/kg bw) and acesulfame-K (1.5, 15 and 150mg/kg bw) by gavage. Bone marrow cells isolated from femora were analyzed for chromosome aberrations. Statistical analysis of the results show that aspartame in combination with acesulfame-K is not significantly genotoxic. /Acesulfame K/ /The authors/ ... investigated the ability of zinc sulfate (5, 25, 50 mM) to inhibit the sweetness of 12 chemically diverse sweeteners, which were all intensity matched to 300 mM sucrose [800 mM glucose, 475 mM fructose, 3.25 mM aspartame, 3.5 mM saccharin, 12 mM sodium cyclamate, 14 mM acesulfame-K, 1.04 M sorbitol, 0.629 mM sucralose, 0.375 mM neohesperidin dihydrochalcone (NHDC), 1.5 mM stevioside and 0.0163 mM thaumatin]. Zinc sulfate inhibited the sweetness of most compounds in a concentration dependent manner, peaking with 80% inhibition by 50 mM. Curiously, zinc sulfate never inhibited the sweetness of Na-cyclamate. This suggests that Na-cyclamate may access a sweet taste mechanism that is different from the other sweeteners, which were inhibited uniformly (except thaumatin) at every concentration of zinc sulfate. We hypothesize that this set of compounds either accesses a single receptor or multiple receptors that are inhibited equally by zinc sulfate at each concentration. The purpose of the present study was to determine the effect of repeated presentation of the same sweet stimulus on sweetness intensity ratings. The sweet stimuli tested in this study were binary and ternary blends of 14 sweeteners that varied widely in chemical structure. A trained panel evaluated the sweetness intensity over four sips of a given mixture presented at 30 s intervals. The individual components in the binary sweetener combinations were intensity-anchored with 5% sucrose, while the individual sweeteners in the ternary mixtures were intensity-anchored with 3% sucrose .... Each self-mixture was also evaluated (e.g. acesulfame-K-acesulfame-K). The main finding of this study was that mixtures consisting of two or three different sweeteners exhibited less reduction in sweetness intensity over four repeated sips than a single sweetener at an equivalent sweetness level. Furthermore, ternary combinations tended to be slightly more effective than binary combinations at lessening the effect of repeated exposure to a given sweet stimulus. These findings suggest that the decline in sweetness intensity experienced over repeated exposure to a sweet stimulus could be reduced by the blending of sweeteners. Human bitter taste is mediated by the hTAS2R family of G protein-coupled receptors. ... /This study/ employed a high-throughput screening approach to discover a novel bitter receptor antagonist (GIV3727) that inhibits activation of hTAS2R31 (formerly hTAS2R44) by saccharin and acesulfame K, two common artificial sweeteners. Pharmacological analyses revealed that GIV3727 likely act/ed/ as an orthosteric, insurmountable antagonist of hTAS2R31. Surprisingly, ... /it was/ also found that this compound could inhibit five additional hTAS2Rs, including the closely related receptor hTAS2R43. Molecular modeling and site-directed mutagenesis studies suggest/ed/ that two residues in helix 7 are important for antagonist activity in hTAS2R31 and hTAS2R43. In human sensory trials, GIV3727 significantly reduced the bitterness associated with the two sulfonamide sweeteners, indicating that hTAS2R antagonists ... /were/ active in vivo. Non-Human Toxicity Values LD50 Rat oral 7431 mg/kg /Acesulfame K/ LD50 Rat ip 2243 mg/kg /Acesulfame K/ |
| 参考文献 |
[1]. BioCong WN, et al. Long-term artificial sweetener acesulfame potassium treatment alters neurometabolic functions in C57BL/6J mice. PLoS One. 2013 Aug 7;8(8):e70257.chemical Assay Reagents
|
| 其他信息 |
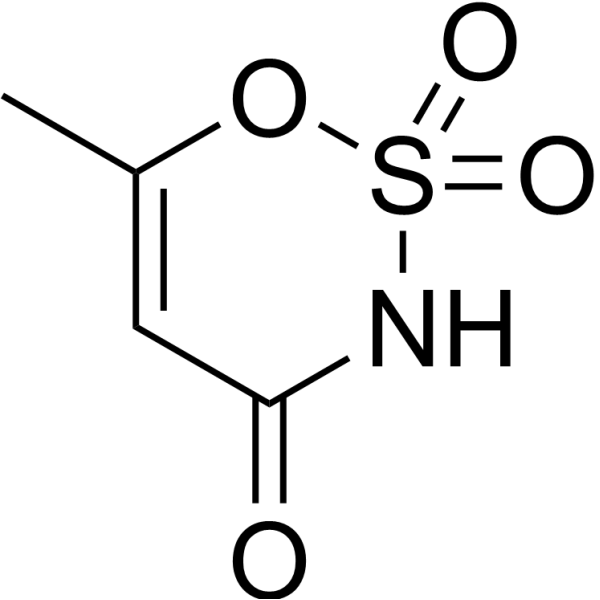
Acesulfame is a sulfamate ester that is 1,2,3-oxathiazin-4(3H)-one 2,2-dioxide substituted by a methyl group at position 6. It has a role as a xenobiotic, an environmental contaminant and a sweetening agent. It is a sulfamate ester, an organonitrogen heterocyclic compound, an oxacycle and an organic heteromonocyclic compound.
|
| 分子式 |
C4H5NO4S
|
|---|---|
| 分子量 |
163.15
|
| 精确质量 |
162.993
|
| CAS号 |
33665-90-6
|
| 相关CAS号 |
Acesulfame potassium;55589-62-3
|
| PubChem CID |
36573
|
| 外观&性状 |
Needles from benzene or chloroform
|
| 密度 |
1.7±0.1 g/cm3
|
| 沸点 |
332.7±25.0 °C at 760 mmHg
|
| 熔点 |
123.2 °C
MP: 225 °C /Acesulfame potatssium/ 123 - 123.5 °C |
| 闪点 |
155.0±23.2 °C
|
| 蒸汽压 |
0.0±1.6 mmHg at 25°C
|
| 折射率 |
1.609
|
| LogP |
-0.31
|
| tPSA |
80.85
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
0
|
| 重原子数目 |
10
|
| 分子复杂度/Complexity |
283
|
| 定义原子立体中心数目 |
0
|
| SMILES |
[K+].CC1=CC(=O)N=S(=O)([O-])O1
|
| InChi Key |
YGCFIWIQZPHFLU-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C4H5NO4S/c1-3-2-4(6)5-10(7,8)9-3/h2H,1H3,(H,5,6)
|
| 化学名 |
6-methyl-2,2-dioxooxathiazin-4-one
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 6.1293 mL | 30.6466 mL | 61.2933 mL | |
| 5 mM | 1.2259 mL | 6.1293 mL | 12.2587 mL | |
| 10 mM | 0.6129 mL | 3.0647 mL | 6.1293 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。