| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
|
| 靶点 |
CSF1R/colony stimulating factor 1 receptor
|
|---|---|
| 体外研究 (In Vitro) |
一种具有式(1)结构的N-(氮杂芳基)环内酰胺-1-甲酰胺衍生物、其制备方法及其用途,每个取代基在说明书和权利要求书中进行了定义。该化合物可广泛应用于制备用于治疗癌症、肿瘤、自身免疫性疾病、代谢性疾病或转移性疾病的药物,特别是用于治疗卵巢癌症、胰腺癌症、前列腺癌症、乳腺癌症、宫颈癌症、胶质母细胞瘤、多发性骨髓瘤、代谢性病变、神经变性疾病、原发肿瘤部位转移或骨转移性癌症的药物,并有望开发成CSF1R抑制剂药物[1]。
|
| 体内研究 (In Vivo) |
腱鞘巨细胞瘤(TGCT)是一种罕见的局部侵袭性软组织肿瘤,起源于关节滑膜、法氏囊和腱鞘,与集落刺激因子1(CSF-1)基因的过表达有关。吡咪考替尼是一种口服、高选择性和强效的小分子CSF-1受体(CSF-1R)抑制剂,对TGCT患者具有强大的疗效和安全性,目前正在开发用于多种疾病。在一项针对不适合手术的TGCT患者的开放标签I期研究中,匹米考替尼显示出优越的疗效和安全性。在这篇文章中,我们阐明了多区域III期MANEUVER试验(NCT05804045)的基本原理和研究设计,该试验旨在评估吡咪考替尼在亚洲、北美和欧洲不适合手术切除的TGCT患者中的疗效和安全性[2]。
|
| 动物实验 |
Randomization & Blinding [2]
Approximately 90 eligible participants will be randomized in a 2:1 ratio to pimicotinib 50 mg QD or matching placebo (Figure 1). Randomization will be conducted across all sites using a central interactive web response system and will be stratified by China sites and non-China sites. In Part 1, treatment assignment will remain blinded to participants, investigators, study site personnel, safety laboratory personnel, central imaging readers and reviewers, as well as the sponsor. After all participants complete Part 1 and reach the primary efficacy analysis time point, unblinding (for the sponsor only) and analysis will be conducted following data cleaning and database lock. Study interventions [2] Pimicotinib or matching placebo is administered QD with a dose of 50 mg in Part 1, and the drug can be taken with or without food. Participants will continue with the same dosage in Part 2 (and Part 3, if applicable) as they were receiving at the conclusion of Part 1. The study drug may be interrupted or dose reduced at the investigator's discretion at any time, e.g., due to AEs. A reduction to 25 mg QD is permissible as the maximum dose adjustment. If a participant requires a therapeutic dose below 25 mg QD, they must discontinue from the treatment. Safety & AEs [2] An AE is defined as any untoward medical occurrence, regardless of its causal relationship to the investigational drug, that occurs in a participant from the time of signing the informed consent form until 30 days (including Day 30) after the last dose of study drug. A treatment-emergent adverse event (TEAE) is defined as any adverse event that occurs or worsens after the initiation of treatment in this study, up to 30 days after the last dose of the study drug, following guidance from the US FDA. The severity of AEs will be graded by the investigator according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0. |
| 参考文献 |
|
| 其他信息 |
Pimicotinib is an orally bioavailable inhibitor of colony stimulating factor 1 receptor (CSF1R; CSF-1R; CD115; M-CSFR), with potential immunomodulatory and antineoplastic activities. Upon oral administration, pimicotinib targets and binds to CSF1R, thereby blocking CSF1R activation and CSF1R-mediated signaling. This inhibits the activities of tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs), and prevents immune suppression in the tumor microenvironment (TME). This enhances antitumor T-cell immune responses and inhibits the proliferation of tumor cells. CSF1R, also known as macrophage colony-stimulating factor receptor (M-CSFR) and CD115 (cluster of differentiation 115), is a cell-surface receptor that plays major roles in tumor cell proliferation and metastasis.
Tenosynovial giant cell tumor (TGCT) is a rare soft tissue tumor which grows in the soft tissues around joints or parts of the body used for movement. It is caused by high levels of a type of protein called CSF-1. Even though surgery is a preferred treatment option, some patients may be unable to have surgery because of where the tumor is, how complicated it is, or the risk of serious problems or illness after the surgery. Therefore, new treatments that are safe, effective and that help people live well are still needed for this disease. Pimicotinib is a medicine which blocks CSF-1, and researches have shown that it is safe and effective for treating TGCT in smaller, early study. To confirm these results, researchers have started a larger study, known as MANEUVER, in some parts of Asia, North America and Europe. This study will confirm if pimicotinib is safe and effective in patients with TGCT who may not be able to have surgery.Clinical Trial Registration: NCT05804045 (ClinicalTrials.gov).[2] The MANEUVER study is structured around a robust three-part design, initiating with a blinded assessment of efficacy and safety, followed by an extended open-label phase that allows for longer observation of secondary end points. Notably, the trial incorporates a placebo crossover component, which is anticipated to strengthen the evidence supporting the efficacy and safety of pimicotinib beyond part 1. Besides objective imaging assessment, most key secondary analyses will focus on several clinical outcome assessments. These measures are critical for demonstrating that pimicotinib offers significant clinical benefits. Furthermore, it is imperative to confirm the absence of conventional hepatotoxicity associated with pimicotinib based on the data from Phase I study. The study's outcomes are expected to clarify pimicotinib's role as a therapeutic option for patients requiring interventions to preserve physical function and enhance quality of life. Most importantly, these results will provide a deeper understanding of disease characteristics and potential variations in response across different populations, i.e. the Chinese and western population. This is the first global trial to enroll both Asian and Western patients with TGCT in balanced proportions across multiple regions. Specifically, Asian participants, primarily from China, constitute half of our study population. This robust representation allows for detailed outcome comparisons by stratification factor – China sites versus non-China sites – thereby facilitating a deeper understanding of disease characteristics and potential variations in response across different populations. While previous research has documented similar anatomical distributions between Chinese and Western patient populations with TGCT, notable disparities in the mean duration from disease onset to diagnosis and histories of previous trauma have been observed [Citation15,Citation16]. This will address critical gaps in existing literature and advance our comprehension of the clinical landscape of TGCT in varied demographic settings.[2] |
| 分子式 |
C22H24N6O3
|
|---|---|
| 分子量 |
420.464364051819
|
| 精确质量 |
420.19
|
| 元素分析 |
C, 62.84; H, 5.75; N, 19.99; O, 11.42
|
| CAS号 |
2253123-16-7
|
| 相关CAS号 |
2866305-19-1 (HCl); 2253123-16-7
|
| PubChem CID |
139549388
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
2
|
| tPSA |
102
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
31
|
| 分子复杂度/Complexity |
674
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
NXFPMDWYDKHFMM-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C22H24N6O3/c1-14-18(31-16-7-9-23-17(11-16)15-12-24-27(4)13-15)5-6-19(25-14)26-21(30)28-10-8-22(2,3)20(28)29/h5-7,9,11-13H,8,10H2,1-4H3,(H,25,26,30)
|
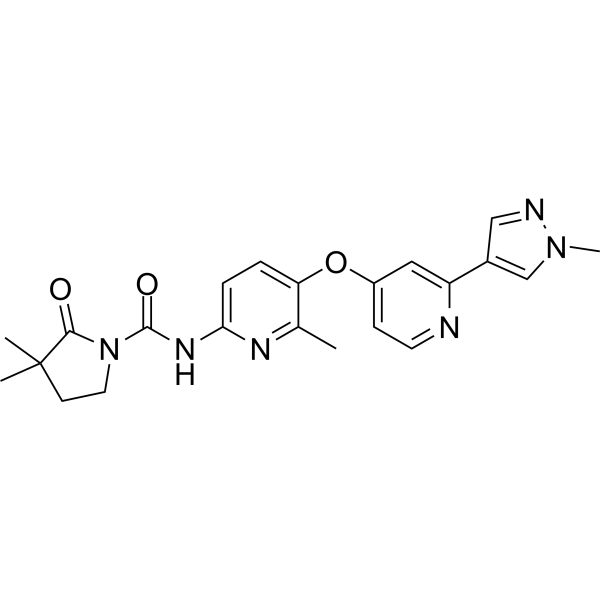
| 化学名 |
3,3-dimethyl-N-[6-methyl-5-[2-(1-methylpyrazol-4-yl)pyridin-4-yl]oxypyridin-2-yl]-2-oxopyrrolidine-1-carboxamide
|
| 别名 |
pimicotinib [INN]; Pimicotinib (USAN/INN); HV1XI8HST2; ABSK021; ABSK-021; CHEMBL5314535;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3783 mL | 11.8917 mL | 23.7835 mL | |
| 5 mM | 0.4757 mL | 2.3783 mL | 4.7567 mL | |
| 10 mM | 0.2378 mL | 1.1892 mL | 2.3783 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。