| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
- μ-opioid receptor (MOR) (Ki = 0.08–0.15 nM, competitive antagonist) [2][3][6]
- κ-opioid receptor (KOR) (Ki = 0.4–0.8 nM, competitive antagonist) [2][3] - δ-opioid receptor (DOR) (Ki = 2.0–3.5 nM, weak competitive antagonist) [2][3] - No significant binding to non-opioid receptors (e.g., GABAₐ, NMDA) at concentrations ≤10 μM [3][6] |
|---|---|
| 体外研究 (In Vitro) |
1. 低剂量抗肿瘤活性:
- 在人乳腺癌细胞系(MCF-7、MDA-MB-231)中,纳曲酮(1–10 nM)通过下调NF-κB活性抑制细胞增殖,抑制率达20–35%;Western blot显示p65磷酸化水平(Ser536)降低40–50%[1]
- 在黑色素瘤细胞(A375)中,纳曲酮(5 nM)通过激活caspase-9诱导凋亡,72小时后凋亡率从对照组的4%升至18%(Annexin V/PI染色法)[1] 2. 阿片受体拮抗活性: - 在稳定表达MOR的CHO细胞中,纳曲酮(0.1–1 nM)以剂量依赖性方式阻断[³H]-二氢吗啡结合,0.12 nM时抑制率达50%(放射性配体置换实验)[3][6] - 在表达KOR的SH-SY5Y神经母细胞瘤细胞中,纳曲酮(0.5 nM)抑制KOR激动剂U50,488H诱导的Ca²⁺内流,抑制率达80%(荧光Ca²⁺成像法)[3] |
| 体内研究 (In Vivo) |
1. 低剂量肿瘤生长抑制:
- 在荷MCF-7乳腺癌异种移植瘤的裸鼠中,口服纳曲酮(0.1 mg/kg,每日1次,连续28天)使肿瘤体积缩小40%,肿瘤重量降低35%(对照组:1.8 ± 0.3 g;纳曲酮组:1.2 ± 0.2 g);免疫组化显示增殖标志物Ki-67阳性率从60%降至30%[1]
- 在荷B16-F10黑色素瘤的C57BL/6小鼠中,纳曲酮(0.05 mg/kg,腹腔注射,隔天1次)使肺转移结节数减少25%(对照组:42 ± 6个;纳曲酮组:32 ± 5个)[1] 2. 阿片依赖逆转: - 在吗啡诱导的物理依赖大鼠中,皮下注射纳曲酮(1 mg/kg)15分钟内诱发戒断症状(如爪震颤、湿狗样抖动),60分钟时症状最严重(行为评分:8/10,对照组:1/10)[3] - 在训练自服海洛因的恒河猴中,口服纳曲酮(3 mg/kg,每日1次)14天内使海洛因自服量减少70%(对照组:25 ± 4次/天;纳曲酮组:7 ± 2次/天)[2] 3. 酒精依赖缓解: - 在长期摄入10%乙醇的C57BL/6小鼠中,口服纳曲酮(2 mg/kg,每日1次)使乙醇摄入量减少55%(对照组:12 ± 2 g/kg/天;纳曲酮组:5.4 ± 1.1 g/kg/天)[6] - 在酒精诱导条件性位置偏爱(CPP)的大鼠中,纳曲酮(1.5 mg/kg,腹腔注射)阻断CPP表达,偏爱评分从对照组的45 ± 5降至10 ± 3[6] 4. 减重(与安非他酮联用): - 在饮食诱导肥胖(DIO)的Sprague-Dawley大鼠中,口服纳曲酮(3 mg/kg)+安非他酮(10 mg/kg)(每日1次,连续4周)使体重降低12%(对照组:520 ± 20 g;联用组:458 ± 15 g),脂肪量减少18%[5] |
| 酶活实验 |
1. μ-阿片受体结合实验:
- 从表达人MOR的CHO细胞中提取膜蛋白,与[³H]-二氢吗啡(0.5 nM)和纳曲酮(0.01–10 nM)在结合缓冲液(50 mM Tris-HCl,pH 7.4,100 mM NaCl,5 mM MgCl₂)中25°C孵育60分钟。通过玻璃纤维滤膜过滤分离结合配体,液体闪烁计数法检测放射性。实验重复3次,采用Cheng-Prusoff方程计算Ki值[3][6]
2. NF-κB活性实验(抗肿瘤机制): - 用纳曲酮(1–10 nM)处理MCF-7细胞后提取核提取物,与生物素标记的NF-κB共识寡核苷酸在结合缓冲液(20 mM HEPES,pH 7.5,50 mM KCl,1 mM DTT)中4°C孵育30分钟。用链霉亲和素包被板捕获DNA-蛋白复合物,通过抗p65一抗和辣根过氧化物酶(HRP)标记二抗检测NF-κB结合活性,测定450 nm吸光度,活性以对照组为基准归一化[1] |
| 细胞实验 |
1. 肿瘤细胞增殖实验(MTT法):
- 将MCF-7/MDA-MB-231细胞(5×10³个/孔)接种于96孔板,用纳曲酮(0.1–100 nM)处理72小时。加入MTT溶液(0.5 mg/mL),37°C孵育4小时后,用DMSO溶解甲瓒晶体,测定570 nm吸光度。细胞活力相对于对照组计算,MCF-7细胞增殖抑制的IC₅₀为8–10 nM[1]
2. 凋亡实验(Annexin V/PI法): - 将A375黑色素瘤细胞(1×10⁵个/孔)用纳曲酮(5 nM)处理48/72小时,收集细胞并用PBS洗涤,室温避光下用Annexin V-FITC和PI染色15分钟。流式细胞术定量早期(Annexin V⁺/PI⁻)和晚期(Annexin V⁺/PI⁺)凋亡细胞,每组设3个复孔[1] 3. 阿片激动剂诱导Ca²⁺内流实验: - 用Fluo-4 AM(2 μM)在HBSS缓冲液中37°C孵育表达KOR的SH-SY5Y细胞30分钟。用纳曲酮(0.1–1 nM)处理细胞10分钟后,加入KOR激动剂U50,488H(1 μM)。每5秒检测一次荧光强度(激发光488 nm,发射光525 nm),持续5分钟以评估Ca²⁺内流,抑制率相对于仅用U50,488H处理的对照组计算[3] |
| 动物实验 |
1. Breast cancer xenograft model (nude mice):
- Female athymic nude mice (6–8 weeks old) were subcutaneously injected with 1×10⁷ MCF-7 cells (suspended in PBS:Matrigel = 1:1) into the right flank. When tumors reached 100 mm³, mice were randomized to vehicle (0.9% saline, 0.1 mL/10 g) or naltrexone (0.1 mg/kg, dissolved in vehicle) groups. Drugs were administered via oral gavage once daily for 28 days. Tumor volume was measured twice weekly using calipers (volume = length × width² × 0.52), and body weight was recorded weekly. On day 28, mice were euthanized, tumors were excised and weighed, and tumor tissues were fixed in 4% paraformaldehyde for immunohistochemistry [1]
2. Morphine dependence model (rats): - Male Sprague-Dawley rats (250–300 g) were implanted with subcutaneous morphine pellets (75 mg/pellet) once every 72 hours for 14 days to induce physical dependence. On day 15, rats were administered subcutaneous naltrexone (1 mg/kg, dissolved in 0.9% saline) or vehicle. Withdrawal symptoms (paw tremors, wet dog shakes, diarrhea) were scored every 15 minutes for 2 hours using a validated behavioral scale (0 = absent, 2 = severe) [3] 3. Long-acting naltrexone formulation (rhesus monkeys): - Male rhesus monkeys (4–6 kg) trained to self-administer heroin (0.1 mg/kg/infusion) were administered a single intramuscular injection of long-acting naltrexone depot (30 mg/kg, formulated as a microsphere suspension in aqueous buffer). Heroin self-administration was measured daily for 28 days, with infusions recorded via a computerized operant conditioning system. Blood samples were collected weekly to measure plasma naltrexone concentrations [2] 4. Diet-induced obesity model (rats): - Male Sprague-Dawley rats (180–200 g) were fed a high-fat diet (45% kcal from fat) for 8 weeks to induce obesity. Rats were then randomized to vehicle (0.5% methylcellulose), naltrexone (3 mg/kg, dissolved in vehicle), bupropion (10 mg/kg), or combination groups. Drugs were administered via oral gavage once daily for 4 weeks. Body weight was measured weekly, and food intake was recorded daily. At the end of the study, rats were euthanized, and epididymal fat pads were excised and weighed [5] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Although well absorbed orally, naltrexone is subject to significant first pass metabolism with oral bioavailability estimates ranging from 5 to 40%. Both parent drug and metabolites are excreted primarily by the kidney (53% to 79% of the dose), however, urinary excretion of unchanged naltrexone accounts for less than 2% of an oral dose and fecal excretion is a minor elimination pathway. The renal clearance for naltrexone ranges from 30 to 127 mL/min and suggests that renal elimination is primarily by glomerular filtration. 1350 L [intravenous administration] ~ 3.5 L/min [after IV administration] Naltrexone hydrochloride is rapidly and almost completely (about 96%) absorbed from the GI tract following oral administration, but the drug undergoes extensive first-pass metabolism in the liver. Only 5-40% of an orally administered dose reaches systemic circulation unchanged. Considerable interindividual variation in absorption of the drug during the first 24 hours after a single dose has been reported. The bioavailability of naltrexone hydrochloride tablets is reportedly similar to that of an oral solution of the drug (not commercially available in the US). Peak plasma concentrations of naltrexone and 6-beta-naltrexol (the major metabolite of naltrexone) usually occur within 1 hour following oral administration of the tablets and 0.6 hours following oral administration of the solution. Because orally administered naltrexone undergoes substantial first-pass metabolism, plasma concentrations of 6-beta-naltrexol following oral administration are substantially higher than corresponding concentrations of naltrexone. Following oral administration, the area under the serum concentration-time curve (AUC) for 6-beta-naltrexol is 10-30 times greater than the AUC for naltrexone. Following single- or multiple-dose (i.e., once daily) oral administration of naltrexone hydrochloride 50 mg in healthy individuals, peak plasma concentrations of naltrexone and 6-beta-naltrexol averaged 10.6-13.7 and 109-139 ng/mL, respectively. Little, if any, accumulation of naltrexone and/or 6-beta-naltrexol appears to occur following chronic administration of the drug. Following chronic administration of naltrexone, plasma concentrations of 6-beta-naltrexol are at least 40% higher than those following administration of a single dose of the drug; however, plasma concentrations of naltrexone and 6-beta-naltrexol 24 hours after each dose of chronically administered drug are similar to concentrations 24 hours after a single dose of the drug in most patients. Naltrexone hydrochloride is widely distributed throughout the body, but considerable interindividual variation in distribution parameters during the first 24 hours following a single oral dose has been reported. Following subcutaneous administration of radiolabeled drug in rats, the drug distributes into CSF within 30 minutes. In animals, CSF naltrexone concentrations are reported to be approximately 30% of concurrent peak plasma concentrations. The drug and its metabolites have been shown to distribute into saliva and erythrocytes following oral administration in humans. For more Absorption, Distribution and Excretion (Complete) data for Naltrexone (13 total), please visit the HSDB record page. Metabolism / Metabolites Hepatic. When administered orally, naltrexone undergoes extensive biotransformation and is metabolized to 6 beta-naltrexol (which may contribute to the therapeutic effect) and other minor metabolites. Naltrexone is metabolized in the liver principally by reduction of the 6-keto group of naltrexone to 6-beta-naltrexol (6-beta-hydroxynaltrexone). Naltrexone also undergoes metabolism by catechol-O-methyl transferase (COMT) to form 2-hydroxy-3-methoxy-6-beta-naltrexol (HMN) and 2-hydroxy-3-methoxynaltrexone. Several minor metabolites have also been identified, including noroxymorphone and 3-methoxy-6-beta-naltrexol. Because oral but not im administration of naltrexone results in substantial first-pass hepatic metabolism of the drug, 6-beta-naltrexol concentrations following im administration are substantially lower than concentrations of the metabolite obtained following oral administration. Naltrexone does not appear to inhibit or induce its own metabolism following chronic administration. Cytochrome P-450 (CYP) isoenzymes are not involved in the metabolism of naltrexone. Naltrexone and its metabolites undergo conjugation with glucuronic acid. The major fraction of total drug and metabolites in both plasma and urine consists of conjugated metabolites. The drug and its metabolites may undergo enterohepatic circulation. Metabolites of naltrexone may contribute to the opiate antagonist activity of the drug. Like naltrexone, 6-beta-naltrexol is an essentially pure opiate antagonist, with a potency of 6-8% that of naltrexone in precipitating withdrawal symptoms in dogs physically dependent on morphine and 1.25-2% that of naltrexone in mice. Because of its weak affinity for opiate receptors, 2-hydroxy-3-methoxy-6-beta-naltrexol (HMN) may not contribute appreciably to the opiate antagonist activity of naltrexone; however, the in vivo opiate antagonist activity of HMN or 2-hydroxy-3-methoxynaltrexone has not been studied. Noroxymorphone, a minor metabolite of naltrexone, is a potent opiate agonist and may be responsible for the agonist activity (eg, miosis) that occurs infrequently in individuals receiving naltrexone. Naltrexone and its metabolites (unconjugated and conjugated) are excreted principally in urine via glomerular filtration; 6-beta-naltrexol, conjugated 6-beta-naltrexol, and conjugated naltrexone are also excreted via tubular secretion. Naltrexone may also undergo partial reabsorption by the renal tubules. Following single- or multiple-dose oral administration of naltrexone hydrochloride, respectively, approximately 38-60 or 70% of a dose has been recovered in urine, principally as 6-beta-naltrexol (conjugated and unconjugated). Most urinary excretion of naltrexone occurs within the first 4 hours after oral administration. Less than 2% of an orally administered dose is excreted unchanged in urine within 24 hours. Approximately 5-10, 19-35, 7-16, 3.5-4.6, and 0.45% of an oral dose are excreted in urine as conjugated naltrexone, 6-beta-naltrexol, conjugated 6-beta-naltrexol, 2-hydroxy-3-methoxy-6-beta-naltrexol (HMN), and 2-hydroxy-3-methoxynaltrexone, respectively, within 24 hours. Less than 5% of a dose is excreted in feces, principally as 6-beta-naltrexol, within 24 hours following single- or multiple-dose oral administration of the drug. Following oral administration of 50 mg of radiolabeled naltrexone in one patient, approximately 93% of the radiolabeled dose was excreted within 133 hours; about 79 and 14% were excreted in urine and feces, respectively. Following im administration of naltrexone extended-release injection, the half-life of naltrexone and 6-beta-naltrexol is 5-10 days. For more Metabolism/Metabolites (Complete) data for Naltrexone (6 total), please visit the HSDB record page. Naltrexone has known human metabolites that include Naltrexone-3-glucuronide. Hepatic. When administered orally, naltrexone undergoes extensive biotransformation and is metabolized to 6 beta-naltrexol (which may contribute to the therapeutic effect) and other minor metabolites. Route of Elimination: Both parent drug and metabolites are excreted primarily by the kidney (53% to 79% of the dose), however, urinary excretion of unchanged naltrexone accounts for less than 2% of an oral dose and fecal excretion is a minor elimination pathway. The renal clearance for naltrexone ranges from 30 to 127 mL/min and suggests that renal elimination is primarily by glomerular filtration. Half Life: 4 hours for naltrexone and 13 hours for the active metabolite 6 beta-naltrexol. Biological Half-Life 4 hours for naltrexone and 13 hours for the active metabolite 6 beta-naltrexol. Plasma concentrations of naltrexone and 6-beta-naltrexol, the major metabolite, appear to decline in a biphasic manner during the first 24 hours following a single oral dose or during chronic administration of the drug. Following oral administration of single or multiple doses of naltrexone hydrochloride, the plasma half-lives of naltrexone and 6-beta-naltrexol in the initial phase (t1/2 alpha) average 1.1-3.9 and 2.3-3.1 hours, respectively, and the plasma half-lives in the terminal phase (t1/2 beta) average 9.7-10.3 and 11.4-16.8 hours, respectively. Plasma concentrations of naltrexone and 6-beta-naltrexol have also been reported to decline in a triphasic manner following oral administration, with a terminal elimination half-life after the first 24 hours of 96 hours for naltrexone and 18 hours for 6-beta-naltrexol, possibly resulting from initial distribution into body tissues and subsequent redistribution into systemic circulation. Pharmacokinetics of naltrexone hydrochloride (NTX) and naltrexone glucuronide was studied in the dog using HPLC-electrochemical detection with naloxone as internal standard. After iv 5 mg or po 10 mg NTX, ... the elimination half-lives of NTX were 78 +/- 6 min and 74 +/- 6 min, respectively. ... The major metabolite of NTX in dog plasma was beta-glucuronidase-hydrolyzable conjugate. Dosing NTX intravenously and orally, ... the elimination half-lives of the glucuronide from plasma were 3.4 hr and 12.6 hr, respectively. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Naltrexone is a narcotic antagonist. It is also used in the treatment of alcoholism. Naltrexone hydrochloride is designated an orphan drug by the US Food and Drug Administration (FDA) in the maintenance of opiate cessation. HUMAN STUDIES: Naltrexone competes for opiate receptors and displaces opioid drugs from these receptors, thus reversing their effects. It is capable of antagonizing all opiate receptors. The mechanism of action of naltrexone in alcohol dependence is not known. Patients receiving 800 mg of naltrexone hydrochloride daily for up to 1 week in one study showed no evidence of toxicity. However, lower dosages reportedly have been hepatotoxic in some patients. No serious adverse effects were observed following administration of single naltrexone doses of up to 784 mg (as the extended-release im injection) in several healthy individuals. Naltrexone was not associated with the high rates of neonatal mortality or congenital anomalies seen in methadone-exposed neonates. Mutagenic changes and chromosomal damage have occurred in vitro in human lymphocytes exposed to naltrexone. ANIMAL STUDIES: Acute toxicity from naltrexone in mice, rats, and dogs resulted in death secondary to tonic-clonic seizures and/or respiratory failure. Weight loss occurred in monkeys following subcutaneous administration of 100 mg/kg doses, and prostration, seizures, and death occurred following subcutaneous administration of 300 mg/kg doses. Hypoactivity, salivation, and emesis occurred in monkeys following oral administration of 1 g/kg doses, and seizures and death occurred following oral administration of 3 g/kg doses. Bradycardia has occurred following iv naltrexone hydrochloride doses of 5-80 ug/kg in unanesthetized dogs, however, respiratory rate, blood pressure, arterial blood gases, and EEG remained unchanged throughout the dose range. Within 20 minutes of 1 mg/kg iv doses in cats, total brain oxygen consumption decreased by about 48% and blood flow to the entire brain and the pons decreased by about 40%. In a 2-year study of the carcinogenic potential of naltrexone, there was an increase in the frequency of mesotheliomas in male rats and tumors of vascular origin in both male and female rats. No evidence of carcinogenicity was observed in several other 2-year studies in mice or rats receiving naltrexone dosages of 30 or 100 mg/kg daily. Naltrexone dosages of 100 mg/kg daily in rats produced an increase in pseudopregnancy and a decrease in the pregnancy rate in mated rats. Naltrexone did not exhibit clastogenicity in an in-vivo mouse micronucleus assay. No evidence of genotoxic potential was observed in a range of other in-vitro tests, including assays for gene mutation in bacteria, yeast, or in a second mammalian cell line, a chromosomal aberration assay. However, mutagenic changes and chromosomal damage have occurred in vitro in Chinese hamster ovarian cells, in the Drosophila recessive lethal assay, and in nonspecific DNA repair tests with Escherichia coli and WI-38 cells. ECOTOXICITY STUDIES: In order to evaluate the influence of the season (the stage of gonad maturity) on the modulatory role of endogenous opioid peptides in LH secretion in fish, sexually mature male carp (Cyprinus carpio L.) were intravenously injected with naltrexone-opioid receptor antagonist (5 or 50 ug/kg) in the period of natural spawning (June) or gonad recrudescence (December). In June, naltrexone significantly lowered LH levels in comparison to saline injected males. In December, there were no differences between saline and naltrexone-injected carps. Naltrexone is a pure opiate antagonist and has little or no agonist activity. The mechanism of action of naltrexone in alcoholism is not understood; however, involvement of the endogenous opioid system is suggested by preclinical data. Naltrexone is thought to act as a competitive antagonist at mc, kappa, and delta receptors in the CNS, with the highest affintiy for the mu receptor. Naltrexone competitively binds to such receptors and may block the effects of endogenous opioids. This leads to the antagonization of most of the subjective and objective effects of opiates, including respiratory depression, miosis, euphoria, and drug craving. The major metabolite of naltrexone, 6-beta-naltrexol, is also an opiate antagonist and may contribute to the antagonistic activity of the drug. Hepatotoxicity Naltrexone therapy is typically given to patients with a high background rate of liver disease (injection drug use or alcoholism) and has been associated with variable rates of serum enzyme elevations (0% to 50%), values above 3 times the upper limit of normal occurring in approximately 1% of patients and occasionally leading to drug discontinuation. However, several studies have shown that the rate of ALT elevations during naltrexone therapy is similar to that with placebo. Most serum aminotransferase elevations during naltrexone therapy are mild and self-limiting, resolving even with continuation of therapy. While several rare instances of acute, clinically apparent liver disease have been reported in patients taking naltrexone, the role of the medication in the liver injury has not always been clear and there has been no clear description of the clinical features of the injury. Thus, while often considered hepatotoxic, naltrexone has not been definitively linked to cases of clinically apparent liver injury. Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Limited data indicate that naltrexone is minimally excreted into breastmilk. If the mother requires naltrexone, it is not a reason to discontinue breastfeeding. ◉ Effects in Breastfed Infants A 1.5-month-old breastfed infant of a mother who was taking 50 mg of oral naltrexone daily during pregnancy and lactation was reportedly healthy with no naltrexone-related adverse effects. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding 21% bound to plasma proteins over the therapeutic dose range. Toxicity Data LD50: 1,100-1,550 mg/kg (oral, mouse) LD50: 1,450 mg/kg (oral, rat) LD50: 1,490 mg/kg (oral, guinea pig) Interactions Naltrexone may increase the CNS effects of yohimbine (anxiety, tremors, nausea, palpitations) and increase plasma cortisol levels. Naltrexone is a clinically approved medication for alcoholism. We aimed to investigate the effectiveness of naltrexone co-administered with cocaine and the association of these substances with immediate-early gene expression in the rat prefrontal cortex. We used chronic operant ethanol self-administration and oral treatments prescribed for alcoholism and available in pharmacies to maximize the predictive validity in humans. We performed real-time PCR analysis to determine gene expression levels in the prefrontal cortex. Only the highest dose of naltrexone (1, 3, and 10 mg/kg, p.o.) reduced the response to ethanol. Cocaine increased ethanol self-administration in a dose-dependent manner (2.5, 10, 20 mg/kg, i.p.) and reversed the naltrexone-induced reduction. Naltrexone failed to prevent the cocaine-induced increase in locomotor activity observed in these animals. Chronic self-administration of ethanol reduced the expression of the C-fos gene 4- to 12-fold and increased expression of the COX-2 (up to 4-fold) and Homer1a genes in the rat prefrontal cortex. Chronic ethanol self-administration is prevented by naltrexone, but cocaine fully reverses this effect. This result suggests that cocaine may overcome naltrexone's effectiveness as a treatment for alcoholism. The ethanol-induced reduction in C-fos gene expression in the prefrontal cortex reveals an abnormal activity of these neurons, which may be relevant in the compulsive consumption of ethanol, the control of reward-related areas and the behavioral phenotype of ethanol addiction. In appetite research, drugs frequently progress to clinical trials on the basis of outcome (reduced food intake/body weight gain) with insufficient attention to process (behavioral analysis). Although bupropion and naltrexone (alone and in combination) reduce food consumption in rodents and humans, their effects on behavior during feeding tests have not been thoroughly investigated. This study aimed to assess the behavioral specificity of anorectic responses to bupropion, naltrexone and their combination. Video analysis was employed to characterize the behavioral effects of acute systemic treatment with bupropion (10.0-40.0 mg/kg), naltrexone (0.1-3.0 mg/kg) and combined bupropion (20 mg/kg) plus naltrexone (0.1-1.0 mg/kg) in non-deprived male rats exposed for 1 hr to palatable mash. Particular attention was paid to the behavioral satiety sequence (BSS). In experiment 1, the anorectic response to 40 mg/kg bupropion was associated with significant psychomotor stimulation and a complete disruption of the BSS. In experiment 2, the anorectic response to 3 mg/kg naltrexone was associated with an accelerated but otherwise normal BSS. In experiment 3, the co-administration of 20 mg/kg bupropion and naltrexone (0.1 and 1.0 mg/kg) not only produced an additive anorectic profile (including a reduced rate of eating), but the addition of the opioid receptor antagonist also concurrently attenuated the psychomotor stimulant response to the atypical antidepressant. Low-dose co-treatment with naltrexone and bupropion produces a stronger suppression of appetite than that seen with either agent alone and has the additional advantage of reducing some of the unwanted effects of bupropion. Opioid antagonists (e.g., naltrexone) and positive modulators of gamma-aminobutyric-acidA (GABAA) receptors (e.g., alprazolam) modestly attenuate the abuse-related effects of stimulants like amphetamine. The use of higher doses to achieve greater efficacy is precluded by side effects. Combining naltrexone and alprazolam might safely maximize efficacy while avoiding the untoward effects of the constituent compounds. The present pilot study tested the hypothesis that acute pretreatment with the combination of naltrexone and alprazolam would not produce clinically problematic physiological effects or negative subjective effects and would reduce the positive subjective effects of d-amphetamine to a greater extent than the constituent drugs alone. Eight nontreatment-seeking, stimulant-using individuals completed an outpatient experiment in which oral d-amphetamine (0, 15, and 30 mg) was administered following acute pretreatment with naltrexone (0 and 50 mg) and alprazolam (0 and 0.5 mg). Subjective effects, psychomotor task performance, and physiological measures were collected. Oral d-amphetamine produced prototypical physiological and stimulant-like positive subjective effects (e.g., VAS ratings of Active/Alert/Energetic, Good Effect, and High). Pretreatment with naltrexone, alprazolam, and their combination did not produce clinically problematic acute physiological effects or negative subjective effects. Naltrexone and alprazolam each significantly attenuated some of the subjective effects of d-amphetamine. The combination attenuated a greater number of subjective effects than the constituent drugs alone. The present results support the continued evaluation of an opioid receptor antagonist combined with a GABAA-positive modulator using more clinically relevant experimental conditions like examining the effect of chronic dosing with these drugs on methamphetamine self-administration. For more Interactions (Complete) data for Naltrexone (7 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Mouse oral 1.1-1.55 g/kg LD50 Rat oral 1.45 g/kg LD50 Guinea pig oral 1.49 g/kg LD50 Monkey oral 3.0 g/kg For more Non-Human Toxicity Values (Complete) data for Naltrexone (8 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
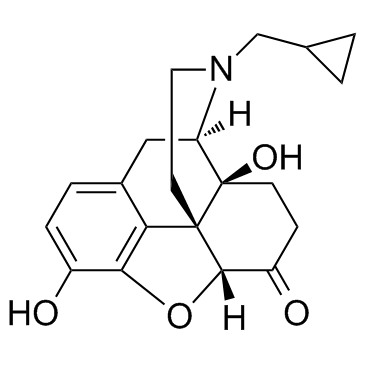
Naltrexone is an organic heteropentacyclic compound that is naloxone substituted in which the allyl group attached to the nitrogen is replaced by a cyclopropylmethyl group. A mu-opioid receptor antagonist, it is used to treat alcohol dependence. It has a role as a mu-opioid receptor antagonist, a central nervous system depressant, an environmental contaminant, a xenobiotic and an antidote to opioid poisoning. It is an organic heteropentacyclic compound, a morphinane-like compound and a member of cyclopropanes. It is a conjugate base of a naltrexone(1+).
Derivative of noroxymorphone that is the N-cyclopropylmethyl congener of naloxone. It is a narcotic antagonist that is effective orally, longer lasting and more potent than naloxone, and has been proposed for the treatment of heroin addiction. The FDA has approved naltrexone for the treatment of alcohol dependence. Naltrexone is an Opioid Antagonist. The mechanism of action of naltrexone is as an Opioid Antagonist. Naltrexone is a synthetic opioid antagonist used in prevention of relapse of opiate addiction and alcoholism. Naltrexone has been associated with low rates of serum enzyme elevations during therapy and with rare instances of clinically apparent liver injury. Naltrexone is a noroxymorphone derivative with competitive opioid antagonistic property. Naltrexone reverses the effects of opioid analgesics by binding to the various opioid receptors in the central nervous system, including the mu-, kappa- and gamma-opioid receptors. This leads to an inhibition of the typical actions of opioid analgesics, including analgesia, euphoria, sedation, respiratory depression, miosis, bradycardia, and physical dependence. Naltrexone is longer-acting and more potent compared to naloxone. Derivative of noroxymorphone that is the N-cyclopropylmethyl congener of naloxone. It is a narcotic antagonist that is effective orally, longer lasting and more potent than naloxone, and has been proposed for the treatment of heroin addiction. The FDA has approved naltrexone for the treatment of alcohol dependence. [PubChem] Derivative of noroxymorphone that is the N-cyclopropylmethyl congener of NALOXONE. It is a narcotic antagonist that is effective orally, longer lasting and more potent than naloxone, and has been proposed for the treatment of heroin addiction. The FDA has approved naltrexone for the treatment of alcohol dependence. See also: Naltrexone Hydrochloride (has salt form). Drug Indication Used as an adjunct to a medically supervised behaviour modification program in the maintenance of opiate cessation in individuals who were formerly physically dependent on opiates and who have successfully undergone detoxification. Also used for the management of alcohol dependence in conjunction with a behavioural modification program. FDA Label Mechanism of Action Naltrexone is a pure opiate antagonist and has little or no agonist activity. The mechanism of action of naltrexone in alcoholism is not understood; however, involvement of the endogenous opioid system is suggested by preclinical data. Naltrexone is thought to act as a competitive antagonist at mc, κ, and δ receptors in the CNS, with the highest affintiy for the μ receptor. Naltrexone competitively binds to such receptors and may block the effects of endogenous opioids. This leads to the antagonization of most of the subjective and objective effects of opiates, including respiratory depression, miosis, euphoria, and drug craving. The major metabolite of naltrexone, 6-β-naltrexol, is also an opiate antagonist and may contribute to the antagonistic activity of the drug. Naltrexone competes for opiate receptors and displaces opioid drugs from these receptors, thus reversing their effects. It is capable of antagonizing all opiate receptors. Therapeutic Uses Narcotic Antagonists /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Naltrexone is included in the database. Naltrexone hydrochloride is designated an orphan drug by the US Food and Drug Administration (FDA) and is used orally for its opiate antagonist effects as an adjunct to a medically supervised behavior modification program in the maintenance of opiate cessation (opiate-free state) in individuals formerly physically dependent on opiates and who have successfully undergone detoxification. /Included in US product label/ Naltrexone is used orally or im in the management of alcohol dependence in conjunction with a comprehensive management program that includes psychosocial support. /Included in US product label/ For more Therapeutic Uses (Complete) data for Naltrexone (31 total), please visit the HSDB record page. Drug Warnings Naltrexone hydrochloride is contraindicated in: 1. Patients receiving opioid analgesics. 2. Patients currently dependent on opioids, including those currently maintained on opiate agonists (e.g., methadone ) or partial agonists (e.g., buprenorphine). 3. Patients in acute opioid withdrawal. 4. Any individual who has failed the naloxone challenge test or who has a positive urine screen for opioids. 5. Any individual with a history of sensitivity to naltrexone hydrochloride or any other components of this product. It is not known if there is any cross-sensitivity with naloxone or the phenanthrene containing opioids. Cases of hepatitis and clinically significant liver dysfunction were observed in association with naltrexone hydrochloride exposure during the clinical development program and in the postmarketing period. Transient, asymptomatic hepatic transaminase elevations were also observed in the clinical trials and postmarketing period. When patients presented with elevated transaminases, there were often other potential causative or contributory etiologies identified, including pre-existing alcoholic liver disease, hepatitis B and/or C infection, and concomitant usage of other potentially hepatotoxic drugs. Although clinically significant liver dysfunction is not typically recognized as a manifestation of opioid withdrawal, opioid withdrawal that is precipitated abruptly may lead to systemic sequelae, including acute liver injury. Patients should be warned of the risk of hepatic injury and advised to seek medical attention if they experience symptoms of acute hepatitis. Use of naltrexone hydrochloride should be discontinued in the event of symptoms and/or signs of acute hepatitis. An increase in naltrexone AUC of approximately 5- and 10-fold in patients with compensated and decompensated liver cirrhosis, respectively, compared with subjects with normal liver function has been reported. These data also suggest that alterations in naltrexone bioavailability are related to liver disease severity. Studies to evaluate possible interactions between naltrexone hydrochloride and drugs other than opiates have not been performed. Consequently, caution is advised if the concomitant administration of naltrexone hydrochloride and other drugs is required. The safety and efficacy of concomitant use of naltrexone hydrochloride and disulfiram is unknown, and the concomitant use of two potentially hepatotoxic medications is not ordinarily recommended unless the probable benefits outweigh the known risks. Lethargy and somnolence have been reported following doses of naltrexone hydrochloride and thioridazine. Patients taking naltrexone hydrochloride may not benefit from opioid containing medicines, such as cough and cold preparations, antidiarrheal preparations, and opioid analgesics. In an emergency situation when opioid analgesia must be administered to a patient receiving naltrexone hydrochloride, the amount of opioid required may be greater than usual, and the resulting respiratory depression may be deeper and more prolonged. For more Drug Warnings (Complete) data for Naltrexone (36 total), please visit the HSDB record page. Pharmacodynamics Naltrexone, a pure opioid antagonist, is a synthetic congener of oxymorphone with no opioid agonist properties. Naltrexone is indicated in the treatment of alcohol dependence and for the blockade of the effects of exogenously administered opioids. It markedly attenuates or completely blocks, reversibly, the subjective effects of intravenously administered opioids. When co-administered with morphine, on a chronic basis, naltrexone blocks the physical dependence to morphine, heroin and other opioids. In subjects physically dependent on opioids, naltrexone will precipitate withdrawal symptomatology. - Background: Naltrexone is a synthetic opioid receptor antagonist approved by the FDA in 1984 for opioid dependence, 1994 for alcohol dependence, and 2010 (in combination with bupropion) for chronic weight management [2][5][6] - Mechanism of action: - For addiction: Blocks MOR-mediated reward pathways (e.g., mesolimbic dopamine system) to reduce craving and reinforcement of opioid/alcohol use [2][3][6] - For cancer (low-dose): Inhibits NF-κB activation (reduces inflammation and tumor cell proliferation) and modulates immune function (increases natural killer cell activity) [1] - For weight loss (combination): Naltrexone blocks hypothalamic opioid receptors (reduces food craving), while bupropion inhibits dopamine/norepinephrine reuptake (suppresses appetite) [5] - Clinical efficacy: - Opioid dependence: 50 mg/day oral naltrexone reduces relapse rate by 40–50% vs. placebo over 6 months [3] - Alcohol dependence: 50 mg/day oral naltrexone reduces heavy drinking days by 30–40% vs. placebo [6] - Weight loss: Naltrexone (8 mg) + bupropion (90 mg) twice daily reduces body weight by 5–7% vs. placebo at 1 year in obese patients [5] - Cancer (preclinical): Low-dose naltrexone enhances the efficacy of chemotherapy (e.g., paclitaxel) in MCF-7 xenografts (tumor volume reduction increased from 40% to 65% with combination) [1] - Adherence challenges: Oral naltrexone has poor adherence (30–40% at 6 months) in addiction patients due to lack of reinforcing effects; long-acting depot formulations improve adherence to 70–80% [2][4] - FDA warnings: Risk of opioid withdrawal if administered to opioid-dependent patients; avoid use in patients with acute hepatitis or severe liver dysfunction [2][6] |
| 分子式 |
C20H23NO4
|
|---|---|
| 分子量 |
341.40
|
| 精确质量 |
341.162
|
| 元素分析 |
C, 70.36; H, 6.79; N, 4.10; O, 18.75
|
| CAS号 |
16590-41-3
|
| 相关CAS号 |
Naltrexone-d4;2070009-29-7; 16590-41-3 (free);16676-29-2 (HCl);
|
| PubChem CID |
5360515
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 沸点 |
558.1±50.0 °C at 760 mmHg
|
| 熔点 |
168-170ºC
|
| 闪点 |
291.4±30.1 °C
|
| 蒸汽压 |
0.0±1.6 mmHg at 25°C
|
| 折射率 |
1.709
|
| LogP |
1.8
|
| tPSA |
70
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
25
|
| 分子复杂度/Complexity |
621
|
| 定义原子立体中心数目 |
4
|
| SMILES |
C1CC1CN2CC[C@]34[C@@H]5C(=O)CC[C@]3([C@H]2CC6=C4C(=C(C=C6)O)O5)O
|
| InChi Key |
DQCKKXVULJGBQN-XFWGSAIBSA-N
|
| InChi Code |
InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1
|
| 化学名 |
(4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-dihydroxy-2,4,5,6,7a,13-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-7-one
|
| 别名 |
naltrexone; 16590-41-3; Vivitrol; Vivitrex; Celupan; N-Cyclopropylmethylnoroxymorphone; Naltrexona; Naltrexonum;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ≥ 31 mg/mL (90.80 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9291 mL | 14.6456 mL | 29.2912 mL | |
| 5 mM | 0.5858 mL | 2.9291 mL | 5.8582 mL | |
| 10 mM | 0.2929 mL | 1.4646 mL | 2.9291 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
CURE Addiction Center of Excellence: Brain Mechanisms of Relapse and Recovery
CTID: NCT01587196
Phase: Phase 2 Status: Completed
Date: 2024-10-15