| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
β2/1-adrenergic receptor
|
|---|---|
| 体外研究 (In Vitro) |
Zenidolol (ICI-118551) 盐酸盐抑制 IMCD 细胞中 cAMP 的积累,IC50 为 1.7 μM[1]。 Zenidolol (ICI-118551; 10 μM) 盐酸盐可诱导去甲肾上腺素 (NE) 预收缩的 PA 显着血管舒张,但不会引起 AO[2]。对于衰竭的人类心脏,Zenidolol (ICI-118551) 盐酸盐对心跳持续时间有显着影响,与基础收缩相比,收缩峰值时间和舒张至 90% 的时间缩短。 Zenidolol (ICI-118551) 盐酸盐的负性肌力作用与 cAMP 无关。兔肌细胞中 β2AR 的过表达增强了盐酸 Zenidolol (ICI-118551) 的负性肌力作用[3]。
假设ICI 118551结合将β2 AR导向G(i)偶联形式,并远离G(s)偶联形式(配体导向贩运)。ICI 118551有效地充当G(i)偶联β(2)AR的激动剂,产生直接的负性肌力作用。β(2)AR存在且G(i)升高的情况(衰竭的人类心脏、TGbeta(2)小鼠心脏)易出现负性肌力作用[5]。 |
| 体内研究 (In Vivo) |
将盐酸泽尼洛尔(Zenidolol (ICI-118551);0.2 mg/kg)注射到小鼠颈静脉中,可降低肺循环中的收缩压,但不会降低全身动脉压[2]。
1.比较选择性β2肾上腺素受体拮抗剂Zenidolol (ICI-118551)与普萘洛尔对原发性震颤、心率和血压的影响。2.Zenidolol (ICI-118551)(每天150毫克,持续7天)和普萘洛尔(每天120毫克,连续7天)在减少原发性震颤方面的效果大致相同(约40%),并且比安慰剂更有效。3.与安慰剂的效果相比,普萘洛尔降低了血压和运动心率,而ICI 118551对血压没有显著影响,并在运动性心动过速方面产生了微小但显著的降低。4.ICI 118551可用于治疗原发性震颤,同时比非选择性β肾上腺素受体拮抗剂具有更少的心血管副作用。[2] β(2)肾上腺素能受体拮抗剂-(异丙氨基)-1-[(7-甲基-4-茚满基)氧基]丁-2-醇(ICI-118551)(1或2 mg/kg i.p.)通过强迫游泳或BRL-44408阻断了恢复,而非选择性β-肾上腺素能受体激动剂异丙肾上腺素(2或4 mg/kg i.p.m.)或β(2”肾上腺素能受体选择性激动剂克伦特罗(2或4mg/kg i.p.p.)的给药则诱导了恢复。高剂量(20mg/kg)但不低剂量(10mg/kg)的β(1)肾上腺素能受体拮抗剂倍他洛尔也会阻断强迫游泳诱导的恢复,但不会阻断BRL-44408诱导的恢复。异丙肾上腺素诱导的恢复也会被ICI-118551或倍他洛尔预处理阻断,这表明β1和β2肾上腺素能受体在应激诱导的恢复中具有潜在的协同作用。总的来说,这些发现表明,靶向β肾上腺素能受体可能是一种有前景的药物治疗策略,可以预防药物复发,特别是在与压力相关的可卡因成瘾者中。[3] |
| 酶活实验 |
在测定前一小时将生长培养基从孔中取出并用 50 uL Hanks 平衡盐溶液替换。该溶液还包含 0.5 mM MgCl2•6H2O、0.4 mM MgSO4•7H2 O、20 mM N-2-羟乙基哌嗪-N'-2乙磺酸 (HEPES)、1.2 mM 3-异丁基-1-甲基黄嘌呤 (IBMX)、0.95 mM CaCl2 和 0.05% BSA。对于剂量反应研究,将每个板浸入 37°C 的振荡水浴中。在一项研究中,添加了 5 个孔/剂量/板,然后用 10 分钟孵育不同剂量的异丙肾上腺素 (10-9-10-5 M) 和 β1 - 和 β2- 受体选择性部分激动剂(他唑洛尔、普那特罗、沙丁胺醇和特布他林,分别为 10-6 和 10-5 M)。另一项研究使用 10 μM 异丙肾上腺素刺激细胞,使用或不使用不同剂量的 β-肾上腺素受体拮抗剂。 10 分钟后,添加 100 μL 10% 三氯乙酸 (TCA) 停止孵育,最终 TCA 浓度为 5%。使用 H20-饱和乙醚萃取 TCA 两次后,将样品在 80°C 脱水整夜,然后重新悬浮在 50 mM 乙酸钠缓冲液中。使用放射免疫测定试剂盒测定CAMP含量。
|
| 细胞实验 |
为了进行结合反应,将 60 μg 膜与不同浓度的 ICI 118,551 和 10 nM [3H]二氢阿普洛尔盐酸盐一起孵育。在室温下孵育两小时后,通过玻璃纤维过滤器快速过滤混合物来停止结合反应。然后,使用液体闪烁计数器测量过滤器中的放射性。 1 μM 阿普洛尔的存在用于确定非特异性结合。 GraphPad Prism 用于分析结合数据。
研究人员观察到选择性β2肾上腺素受体(AR)拮抗剂Zenidolol (ICI-118551)在衰竭的人类心室肌细胞中的直接(非儿茶酚胺阻断)负性肌力作用。在这项研究中,我们同时描述了人类肌细胞和β(2)AR或G(i)蛋白过表达的动物模型中的肌细胞的作用。 方法和结果:将酶分离的超灌注心室肌细胞暴露于βAR激动剂和拮抗剂/反向激动剂,并测量收缩幅度。Zenidolol (ICI-118551)使衰竭人类心脏的心室肌细胞收缩减少了45.3+/-4.1%(n=20个心脏/31个肌细胞,P<0.001),但对非衰竭心脏的影响很小(4.9+/-4%,n=5个肌细胞/3个心脏)。在被归类为终末期的患者中,效果明显更大。具有高β(2)AR数和增加的G(i)水平的转基因小鼠具有正常的基础收缩性,但对Zenidolol (ICI-118551)表现出类似的负性肌力反应。使用腺病毒在兔心肌细胞中过表达人β(2)AR增强了ICI 118551的负性肌力作用。在人类、兔子和小鼠的肌细胞中,用百日咳毒素处理细胞以灭活G(i)后,负性肌力作用被阻断,G(i)α(2)的过表达在正常大鼠肌细胞中重新诱导了这种作用[5]。 |
| 动物实验 |
Adrenaline may increase noradrenaline release and enhance sympathetic pressor effects through activation of pre-synaptic beta 2-adrenoceptors. Conversely, blockade of beta 2-receptors could lead to a fall in blood pressure. To test this hypothesis we performed a double-blind placebo controlled crossover study in nine patients with mild hypertension, comparing the effects of the beta 2-selective blocker Zenidolol (ICI-118551), 50 mg t.i.d. with those of propranolol, 80 mg t.i.d. Two hours after the first dose of Zenidolol (ICI-118551) or propranolol, plasma noradrenaline and blood pressure remained unchanged while heart rate and renin were reduced. After 1 week, blood pressure was significantly reduced by both drugs. The beta 2-selectivity of ICI 118,551 was confirmed by isoprenaline infusion studies. After 1 week of treatment ICI 118,551 had no effect on the beta 1-receptor mediated shortening of electromechanical systole (QS2I), the rise in systolic pressure and rise in renin, whereas these responses were blocked by a dose factor of eight after propranolol. ICI 118,551 and propranolol equally blocked the beta 2-receptor mediated fall in diastolic pressure and the rise in noradrenaline. We conclude that beta 2-selective blockade by ICI 118,551 lowers blood pressure. This finding is compatible with a role of pre-synaptic beta 2-receptors in blood pressure control.[4]
|
| 参考文献 | |
| 其他信息 |
Although many beta1-receptor antagonists and beta2-receptor agonists have been used in pharmacotherapy for many years their pharmacological properties at all three known subtypes of beta-adrenergic receptors are not always well characterized. The aim of this study was, therefore, to provide comparative binding characteristics of agonists (epinephrine, norepinephrine, isoproterenol, fenoterol, salbutamol, salmeterol, terbutalin, formoterol, broxaterol) and antagonists (propranolol, alprenolol, atenolol, metoprolol, bisoprolol, carvedilol, pindolol, BRL 37344, CGP 20712, SR 59230A, CGP 12177, ICI 118551) at all three subtypes of human beta-adrenergic receptors in an identical cellular background. We generated Chinese hamster ovary (CHO) cells stably expressing the three beta-adrenergic receptor subtypes at comparable levels. We characterized these receptor subtypes and analyzed the affinity of routinely used drugs as well as experimental compounds in competition binding studies, using the non-selective antagonist 125I-cyanopindolol as a radioligand. Furthermore, we analyzed the beta-receptor-mediated adenylyl cyclase activity in isolated membranes from these cell lines. The results from our experiments show that all compounds exhibit distinct patterns of selectivity and activity at the three beta-receptor subtypes. In particular, a number of beta2- or beta3-receptor agonists that are inverse agonists at the other subtypes were identified. In addition, beta1-receptor antagonists with agonistic activity at beta2- and beta3-receptors were found. These specific mixtures of agonism, antagonism, and inverse agonism at different subtypes may have important implications for the therapeutic use of the respective compounds.[1]
β-adrenergic receptors (β-ARs) are model G-protein coupled receptors that mediate signal transduction in the sympathetic nervous system. Despite the widespread clinical use of agents that target β-ARs, the signaling pathways that operate downstream of β-AR stimulation have not yet been completely elucidated. Here, we utilized a lysate microarray approach to obtain a broad-scale perspective of phosphoprotein signaling downstream of β-AR. We monitored the time course of phosphorylation states of 54 proteins after β-AR activation mouse embryonic fibroblast (MEF) cells. In response to stimulation with the non-selective β-AR agonist isoproterenol, we observed previously described phosphorylation events such as ERK1/2(T202/Y204) and CREB(S133), but also novel phosphorylation events such as Cdc2(Y15) and Pyk2(Y402). All of these events were mediated through cAMP and PKA as they were reproduced by stimulation with the adenylyl cyclase activator forskolin and were blocked by treatment with H89, a PKA inhibitor. In addition, we also observed a number of novel isoproterenol-induced protein dephosphorylation events in target substrates of the PI3K/AKT pathway: GSK3β(S9), 4E-BP1(S65), and p70s6k(T389). These dephosphorylations were dependent on cAMP, but were independent of PKA and correlated with reduced PI3K/AKT activity. Isoproterenol stimulation also led to a cAMP-dependent dephosphorylation of PP1α(T320), a modification known to correlate with enhanced activity of this phosphatase. Dephosphorylation of PP1α coincided with the secondary decline in phosphorylation of some PKA-phosphorylated substrates, suggesting that PP1α may act in a feedback loop to return these phosphorylations to baseline. In summary, lysate microarrays are a powerful tool to profile phosphoprotein signaling and have provided a broad-scale perspective of how β-AR signaling can regulate key pathways involved in cell growth and metabolism.[6] |
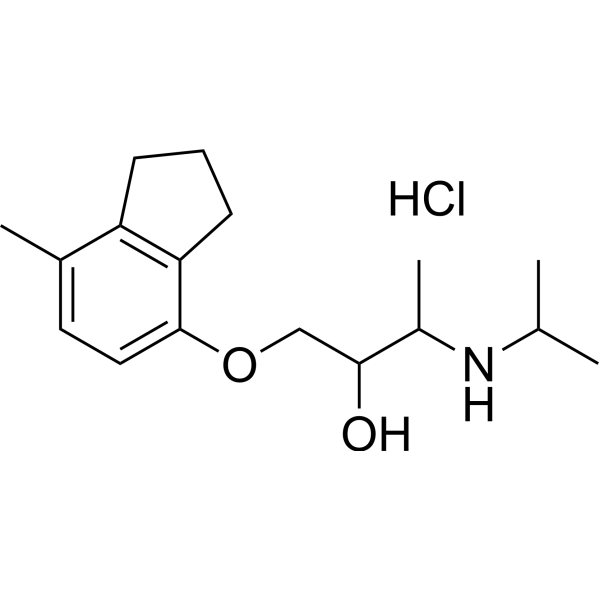
| 分子式 |
C17H28CLNO2
|
|---|---|
| 分子量 |
313.86
|
| 精确质量 |
313.18
|
| 元素分析 |
C, 65.06; H, 8.99; Cl, 11.29; N, 4.46; O, 10.19
|
| CAS号 |
1217094-53-5
|
| 相关CAS号 |
Zenidolol; 72795-26-7; 91021-57-7 (racemic); 72795-01-8; 72795-26-7
|
| PubChem CID |
11957590
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| tPSA |
41.5
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
21
|
| 分子复杂度/Complexity |
295
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O(CC(C(C)NC(C)C)O)C1C=CC(C)=C2CCCC2=1.Cl
|
| InChi Key |
KBXMBGWSOLBOQM-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C17H27NO2.ClH/c1-11(2)18-13(4)16(19)10-20-17-9-8-12(3)14-6-5-7-15(14)17;/h8-9,11,13,16,18-19H,5-7,10H2,1-4H3;1H
|
| 化学名 |
1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy]-3-(propan-2-ylamino)butan-2-ol;hydrochloride
|
| 别名 |
1217094-53-5; ICI 118551 Hydrochloride; 1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy]-3-(propan-2-ylamino)butan-2-ol;hydrochloride; ICI 118,551 hydrochloride; (Rac)-ICI-118551 (hydrochloride); 72795-01-8; ICI-118551; 3-(isopropylamino)-1-((7-methyl-2,3-dihydro-1H-inden-4-yl)oxy)butan-2-ol hydrochloride;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1861 mL | 15.9307 mL | 31.8613 mL | |
| 5 mM | 0.6372 mL | 3.1861 mL | 6.3723 mL | |
| 10 mM | 0.3186 mL | 1.5931 mL | 3.1861 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。