| 规格 | 价格 | ||
|---|---|---|---|
| 500mg | |||
| 1g | |||
| Other Sizes |
| 靶点 |
GABAB receptor
|
|---|---|
| 体外研究 (In Vitro) |
3-氨基丙基次膦酸(10 μM)可作为皮肤抗衰老药物[4]。它还会诱导电刺激回肠中胆碱能抽搐收缩的浓度依赖性抑制(IC50=1.84-0.23 μM)[2]。
测量了GABA、β-丙氨酸和甘氨酸的膦类似物对豚鼠回肠纵肌的影响。3-氨基丙基次膦酸(AMPh)和2-氨基乙基膦酸(2-AEPh)在非刺激制剂和电刺激制剂中均无任何作用。GABA的膦类似物3-Aminopropylphosphinic acid/3-氨基丙基膦酸(3-APPh)在10(-3)M的剂量下具有GABAB激动作用(放松和抑制抽搐反应)。没有观察到对GABAA受体的激动作用。3-APPh在测试剂量(2 X 10(-4)M和10(-3)M)下也对GABAB激动剂的作用显示出拮抗作用,导致GABA和(-)-巴氯芬抑制抽搐反应的对数剂量-效应曲线平行偏移。相比之下,3-APPh没有拮抗吗啡和去甲肾上腺素的抑制作用。通过GABAA受体介导的GABA的收缩作用不受3-APPh(10(-3)M)的影响。结论3-APPh是豚鼠回肠GABAB位点的部分激动剂。[1] 1.测试了γ-氨基丁酸(GABA)类似物3-氨基丙基次膦酸对豚鼠离体回肠和大鼠离体肛尾肌制剂的活性。比较了3-Aminopropylphosphinic acid/3-氨基丙基次膦酸与GABA和巴氯芬的作用。2.在电刺激的回肠中,3-氨基丙基次膦酸,如GABA和巴氯芬,对胆碱能抽搐收缩产生浓度依赖性抑制,IC50值为1.84+/-0.23 microM(n=12)。与GABA不同,但与巴氯芬一样,3-氨基丙基次膦酸不会产生初始收缩。3.3-氨基丙基次膦酸和巴氯芬在豚鼠回肠中的抑制作用未被荷包牡丹碱(10微M)、酚妥拉明加普萘洛尔(均为1微M),育亨宾(1微M。然而,3-氨基丙基次膦酸的抑制作用,而巴氯芬的抑制作用则没有,被phaclofen(500微M)拮抗。此外,巴氯芬脱敏可消除豚鼠回肠中3-氨基丙基次膦酸的影响。4.3-氨基丙基次膦酸、GABA和巴氯芬可减少电场刺激引起的大鼠尾骨肌抽搐收缩。3-氨基丙基次膦酸抑制尾骨收缩的IC50为0.89+/-0.15微M(n=8)。5.结论是,3-氨基丙基次膦酸是一种强效、选择性的GABAB激动剂,在豚鼠回肠中的效力是巴氯芬的七倍,在大鼠肛门括约肌制剂中的效力比巴氯芬强五倍。[2] |
| 体内研究 (In Vivo) |
3-氨基丙基次膦酸(5 mg/kg;静脉注射)通过阻断 GABA 的作用来抑制豚鼠的迷走神经支气管痉挛[3]。
GABA是中枢神经系统中一种已知的抑制性神经递质。最近的研究还表明,GABA存在于包括肺在内的外周组织中。为了阐明GABA在肺中的作用,研究了GABA和选择性GABA激动剂和拮抗剂对豚鼠神经元诱导的气道收缩的影响。在体外,河豚毒素和阿托品抑制了电场刺激(EFS)诱导的气管收缩,表明收缩是由乙酰胆碱的神经元释放介导的。由EFS引起的收缩,而不是由外源性乙酰胆碱引起的收缩被GABA(EC50=4.5微M)和选择性GABA-B激动剂巴氯芬(EC50=9微摩)抑制,但不被GABA-A激动剂麝香醇抑制。巴氯芬的抑制作用不受GABA-A拮抗剂荷包牡丹碱的影响,但被GABA-B拮抗剂3-Aminopropylphosphinic acid/3-氨基丙基次膦酸(3-APPA)(pA2=4.5)和2-羟基乙酰氯芬(pA2=4.1)显著逆转。在体内,麻醉、机械通气豚鼠的迷走神经刺激(5 V,20 Hz,0.5 ms,5 s)引起胆碱能依赖性支气管痉挛,静脉注射GABA(3和10 mg/kg)和巴氯芬(1-10mg/kg)可抑制该痉挛,但麝香醇不能抑制。GABA和巴氯芬对迷走神经支气管痉挛的抑制作用被3-APPA(5mg/kg,静脉注射)阻断,但未被荷包牡丹碱阻断。分别用酚妥拉明或普萘洛尔阻断α肾上腺素能受体和β肾上腺素能受体治疗动物后,对GABA-B激动剂的反应没有改变。GABA和巴氯芬也不会改变静脉注射乙酰甲胆碱引起的支气管痉挛[3]。 |
| 细胞实验 |
“体外”制剂[1]
实验在雄性豚鼠(体重范围300-500g)中进行;动物们被头部的一击所伤;快速去除末端回肠的片段,并将其放置在以下成分(mM)的改良克雷布斯溶液中:KHzP041.3,KCI 3.4,NaCl134.7,CaC12 2.8,MgSO4 0.6,NaHC03 16.3,葡萄糖7.7。通过Paton和Zar(1968)的方法获得了附着有肌间神经丛的回肠纵向肌条。将管段安装在器官浴中,用5%COZ和95%02的混合物起泡,并保持在37°C。必要时,按照Paton(1963)和Paton&VLzi(1969)描述的方法进行电刺激。使用MARB刺激器的两个同轴铂电极施加刺激(持续时间为1msec的最大矩形脉冲的1.5倍,频率为每分钟6次)。在0.5g的静张力下将回肠连接到等距换能器,并在MARB多导生理记录仪上记录反应。给药前,让制剂平衡60分钟。给药量不得超过总浴量的1%(4毫升)。通过在两次给药之间间隔20分钟,可以防止GABA脱敏的发生。事实上,正如我们之前观察到的(Giotti等人,1983a;b),在非刺激和电刺激制剂中,以15-30min的间隔重复亚最大剂量的GABA都会引起相同的效果。 |
| 动物实验 |
Animal/Disease Models: guinea pigs[3]
Doses: 5 mg/kg Route of Administration: IV Experimental Results: Blocked the inhibitory effects of GABA against vagal broncho-spasm. Guinea-pig ileum [2] Male guinea-pigs (300-450g) were killed by a blow to the head and bled out. Segments of ileum of approximately 3 cm in length were removed from an area 10-15cm proximal to the ileo-caecal junction. Preparations were immediately placed in a modified Krebs solution (BUlbring, 1953) which was continually bubbled with 95% 02 and 5% CO2. Segments were freed of their mesenteric attachment and suspended in 10 ml organ baths containing Krebs solution under an isometric tension of 1 g. Isometric contractions to transmural stimulation (Paton, 1954) were recorded with strain gauge transducers and displayed on an Ormed Multitrace pen recorder. Electrical stimulation of preparations was achieved by passing rectangular pulses (duration 0.5 ms; frequency 0.1 Hz; supramaximal voltage (25-35 V)), from a Grass SD11 stimulator via platinum electrodes. Preparations were allowed to equilibrate for 1 h prior to addition of compounds to the organ bath. Rat anococcygeus [2] Male Wistar rats (200-300 g) were killed by a blow to the head and bled out and their anococcygeus muscles removed as previously described (Gillespie, 1972). The muscles were mounted in organ baths (10 ml) containing modified Krebs solution which was continually gassed with 95% 02 and 5% CO2. A resting tension of 0.5 g was applied and the preparations were field stimulated from Grass SD11 stimulators via platinum ring electrodes with the following stimulus parameters: pulse duration 1 ms; frequency 10 Hz; for 1 s. Isometric muscle responses were measured with a strain gauge transducer and displayed on an Ormed Multitrace pen recorder. Typical tension responses generated in either preparation as a result of electrical stimulation were between 2 and 4 g force. Preparations generating less tension were rejected. In both preparations, sequential agonist concentration-response curves were constructed, allowing 30 min between additions of agonist to minimize tachyphylaxis. When antagonists were used, concentration-response curves to 3-Aminopropylphosphinic acid in the presence of the antagonist were constructed after an initial equiliThe following drugs were used: y-amino-n-butyric acid, (±+baclofen, (±)-propranolol (ICI), phentolamine mesylate, yohimbine hydrochloride, naloxone hydrochloride, 8-phenyltheophylline, impromidine oxylate, bicuculline methiodide, phaclofen and 3-Aminopropylphosphinic acid (prepared by the method of Dingwall et al., 1987a, b). With the exception of 8-phenyltheophylline, all compounds were dissolved in distilled water, subsequent dilutions being made in distilled water and compounds were added to the organ bath in volumes no greater than 1% total volume. 8- Phenyltheophylline was made up in 80% methanol/ 2 M NaOH, subsequent dilutions being made in distilled water and all vehicle controls were negative. Propranolol and phentolamine were added directly to the Krebs solution. |
| 参考文献 |
[1]. GABA-related activities of amino phosphonic acids on guinea-pig ileum longitudinal muscle. J Auton Pharmacol. 1986 Sep;6(3):163-9.
[2]. 3-Aminopropylphosphinic acid--a potent, selective GABAB receptor agonist in the guinea-pig ileum and rat anococcygeus muscle. Br J Pharmacol. 1989 Aug;97(4):1292-6. [3]. Prejunctional GABA-B inhibition of cholinergic, neurally-mediated airway contractions in guinea-pigs. Pulm Pharmacol. 1991;4(4):218-24. [4]. 3-Aminopropyl dihydrogen phosphate (3-APPA; 3-aminopropane phosphoric acid); a novel anti-aging substance. Journal of Investigative Dermatology, vol. 4, no. 106, 2015, p. 895. |
| 其他信息 |
A bicumlline-insensitive (possibly GABAB)inhibitory effect of 3-APPh on the firing ofcentral neurones has been previously describedby Bioulac, De Tinguy-Moreaud, Vincent &Neuzil, (1979); moreover quite recently Cates,Li, Yakashe, et al., (1984) have demonstratedthat t h i s compound has some aff~tyfor GABAgbinding sites.Regarding GABAA receptors, none of the phosphonic drugs tested showed agonisticactivity on this subtype of receptors: only 3-APPh contracted the ileum but in a bicuculline-and picrotoxin-insensitive manner and thiscontraction was not desensitized by GABA. Thisineffectivenessof 3-APPh on GABAE,receptors isalso in accordance with previous binding studiesperformed at the central level (Galli, Zilletti,Scotton, Adembri & Giotti, 1980; Cates et al.,1984).However our principal interest was in studyingthe antagonistic effect on GABA receptors ofthese drugs: none of them showed an interferencewith the contraction mediated by GABAA,receptors; on the contrary, 3-APPh at high dosesshowed a significant antagonism against GABABmediated inhibitory effects; the antagonist effectof 3-APPh was found to be reversible and specificfor GABA-ergic drugs; 2-AEPh and 3-APPhwere ineffective.A problem that arises is whether the agonisticaction of 3-APPh on GABAB receptors couldinterfere with its antagonistic properties on thesame receptors through a desensitizationphenomenon. The fact that 3-APPh was alsocapable of promptly reversing the GABAA effect on twitch response (Fig. 6)is in favour of a directantagonistic effect. Moreover 3-APPhantagonism of the GABAB effect appeared to becompetitive for the lower dose tested (2 X10- M): dose-effect curves were in a parallelfashion displaced and the maximum effects werepractically unchanged. On the other hand, for thehighest dose (10-3M) the desensitizationphenomenon might play a role; in fact at thisconcentration dose-effect curves were flattenedwhich might be due to the development ofdesensitization.In conclusion, the phosphonic analogue of GABA, 3-Aminopropylphosphinic acid/3-APPh, shows weak GABABantagonistand weak GABAB agonist properties while it isdevoid of any action (agonistic or antagonistic) onGABAA receptors. This profile is probablyinterpretable as that of a partial agonist and isdifferent from that of other drugs suggested asGABAg antagonists: 3-APS (strong GABAAagonist; weak GABAB agonist and antagonist)(Table 2). Therefore it appears that a usefulGABAB antagonist has yet to be found. However,the exact understanding of the differences ofdrugs acting on GABAB receptors may help intheir use as tools in experimental work and indeveloping more selective GABABantagonists.[1]
There have been several studies on thi requirements for GABAB receptor acth which it would appear that even minor tions of the baclofen molecule result in total loss of activity (Olpe et al., 1980; Krogsgard-Larsen, 1988). To date, no GABAB agonist has been shown to be more potent than baclofen on the in vitro systems described here. 3-Aminopropylphosphinic acid was found to be seven times more potent than racemic baclofen in the guinea-pig ileum and five times more potent than racemic baclofen in the rat anococcygeus muscle. In studies reported by Dingwall et al. (1987a, b), 3-aminopropylphosphinic acid has been reported to have an affinity for the GABAB receptor 1 10,0 .0 that is twenty times more than the affinity of baclo100 1000 fen. It is interesting to compare the structures and HeTIactivities of the phosphinic analogue of GABA, described here as a potent agonist, to the phosphonic analogue which has been described as a weak partial agonist/antagonist (Luzzi et al., 1986), although the only difference in structure between the two is the acidic moiety. 3-Aminopropylphosphinic acid has a distorted tetrahedral arrangement of atoms around the phosphorus, has only one acidic proton and has the negative charge distributed over two oxygen atoms, thus it is similar in many respects 100 1000 to GABA. While 3-aminopropylphosphoninic acid has a near tetrahedral arrangement of atoms around the phosphorus, has two acidic protons (depending agonists in on pH) and has the negative charge distributed over oeus muscle three oxygen atoms. guinea-pig In the guinea-pig ileum, it would appear that 3- I), baclofen aminopropylphosphinic acid is interacting with were 0.5 ms GABA, receptors located prejunctionally on cholinThe mean ergic terminals. Since 3-aminopropylphosphinic acid Sid was sig- did not cause an initial contraction, it is likely to be s for either devoid of GABAA agonist activity. Although final statistically validation of this assumption awaits a more specific 0 values for and potent GABA5 antagonist, the results with a onse curves number of known specific receptor antagonists Iic acidm(l) suggest that 3-aminopropylphosphinic acid is not mieters were interacting with any other class of receptor. Further, pramaximal tissues desensitised to baclofen, no longer responded 3-amino- to 3-aminopropylphosphinic acid. Clonidine, which ,than mean was employed to test the specificity of the GABAB '<0.05). In receptor desensitization, was equieffective both was signifi- before and during GABAB receptor desensitization. for baclofen Phaclofen has been claimed to be a weak but select least four tive GABAB antagonist (Kerr et al., 1987; Dutar & n shown by Nicholl, 1988). We were able to demonstrate inhibition of the 3-aminopropylphosphinic acid responses in the guinea-pig ileum but not those of baclofen. It is unclear why this should be so, although since phaclofen has more recently been claimed to show GABA, antagonist activity in a variety of test systems (Karlsson et al., 1988; Soltesz et al., 1988), it ve structural does support our hypothesis that 3-aminovation from propylphosphinic acid, is interacting with GABA, r manipula- receptors. [2] The time course of the 3-Aminopropylphosphinic acid response in both the guinea-pig ileum and the rat anococcygeus muscle was similar to that of GABA and baclofen. The response to GABAB agonists in the guinea-pig ileum is commonly observed to be transient in nature while in the rat anococcygeus muscle it is more long-lasting (Bowery et al., 1981; Muhyaddin et al., 1982). Our pharmacological investigations support the claims of Dingwall et al. (1987a, b) that 3- aminopropylphosphinic acid is a highly potent, selective GABAB agonist. Further experiments with this compound may shed light on the physiological role of GABAB receptors in the mammalian intestine.[2] |
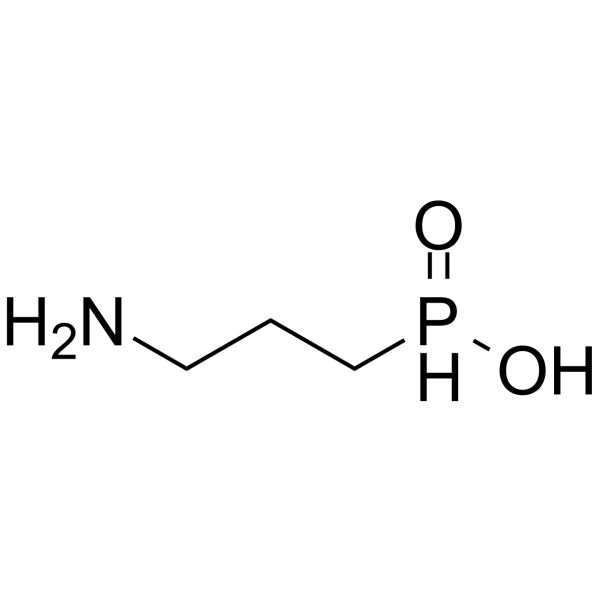
| 分子式 |
C3H10NO2P
|
|---|---|
| 分子量 |
122.08286
|
| 精确质量 |
122.037
|
| CAS号 |
103680-47-3
|
| PubChem CID |
6335948
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 蒸汽压 |
0.001mmHg at 25°C
|
| LogP |
-1.4
|
| tPSA |
95.92
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
7
|
| 分子复杂度/Complexity |
66
|
| 定义原子立体中心数目 |
0
|
| SMILES |
NCCCP(=O)O
|
| InChi Key |
MQIWYGZSHIXQIU-UHFFFAOYSA-O
|
| InChi Code |
InChI=1S/C3H8NO2P/c4-2-1-3-7(5)6/h1-4H2/p+1
|
| 化学名 |
3-aminopropyl-hydroxy-oxophosphanium
|
| 别名 |
3-aminopropylphosphinic acid; 103680-47-3; 3-aminopropyl-hydroxy-oxophosphanium; 3-Aminopropanephosphinic acid; (3-aminopropyl)phosphinic acid; CGP-27492; 3-APPA; Cgp 27492;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 8.1913 mL | 40.9567 mL | 81.9135 mL | |
| 5 mM | 1.6383 mL | 8.1913 mL | 16.3827 mL | |
| 10 mM | 0.8191 mL | 4.0957 mL | 8.1913 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。