| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
GABAC receptor (KB = 2.1 μM)
|
|---|---|
| 体外研究 (In Vitro) |
TPMPA 拮抗 ρ1 受体 (IC50 = 1.6 μM) 和嵌合 ρ1/α1 受体的 GABA 电流,其效力约为 1.3 μM[1]。 TPMPA 针对 rho-1 和 rho-2 受体的 KB 值分别为 2.0 和 15.6 μM,表明活性适中[2]。
此外,特异性GABAC拮抗剂(1,2,5,6-四氢吡啶-4-基)甲基膦酸(TPMPA)拮抗ρ1受体(IC50=1.6μM)和嵌合ρ1/α1受体的GABA电流,其效力大致相同(IC50=1.3μM)(图3B),而GABAA受体仅受到轻微影响。实际上,在锌或TPMPA的高浓度下,GABA的应用会引发外向电流,而不是通常的内向电流(图3)。这可能是因为在高浓度下,锌和TPMPA阻断了特征较差的内向静息电流,并且在GABA对嵌合受体的作用被完全阻断后,这种阻断变得明显[1]。 |
| 细胞实验 |
爪蟾卵母细胞的电生理记录。[1]
卵母细胞的分离和记录基本上如前所述。简而言之,解剖雌性非洲爪蟾的卵巢,手动分离卵泡,为了去除包裹的卵泡细胞,用0.5 mg/ml 1型胶原酶处理卵泡1小时,最后在含有庆大霉素(0.1 mg/ml)的Barth培养基中保持在16°C。一天后,将5-10nl的质粒pcDNAρ1、pcDNAρ1/α1或pcDNAρ1[α1TM2]以0.5mg/ml的浓度注射到爪蟾卵母细胞核中。以相同浓度注射GABAA受体亚基α1β2γ2L(2:2:1)的组合(参见参考文献24)。电生理记录与我们之前描述的记录相似。剂量反应关系用Hill方程拟合(参见参考文献19)。为了估计脱敏率,GABA电流用一个或两个指数衰减函数拟合:τs和τf分别是慢衰减和快衰减分量的时间常数。 |
| 参考文献 |
[1]. GABArho 1/GABAAalpha 1 receptor chimeras to study receptor desensitization. Proc Natl Acad Sci U S A. 2000;97(7):3562-3566.
[2]. Neurologically-active compounds. WO1998058939A1 [3]. GABAc receptors: relatively simple transmitter -gated ion channels?. Trends Pharmacol Sci. 1996;17(9):319-323. |
| 其他信息 |
TPMPA ((1,2,5,6-tetrahydropyridine-4-yl)methylphosphinic acid) is a selective antagonist of GABAC receptors (also known as GABA-ρ or GABAA-ρ receptors). GABAC receptors are found primarily in the retina, although they may be present in other tissues, including the hippocampus, spinal cord, superior colliculus, pituitary and the gut. TPMPA is used as a pharmacological probe for the investigation of GABAC receptor function.
gamma-Aminobutyrate type C (GABA(C)) receptors are ligand-gated ion channels that are expressed preponderantly in the vertebrate retina and are characterized, among other things, by a very low rate of desensitization and resistance to the specific GABA(A) antagonist bicuculline. To examine which structural elements determine the nondesensitizing character of the human homomeric rho1 receptor, we used a combination of gene chimeras and electrophysiology of receptors expressed in Xenopus oocytes. Two chimeric genes were constructed, made up of portions of the rho1-subunit and of the alpha1-subunit of the GABA(A) receptor. When expressed in Xenopus oocytes, one chimeric gene (rho1/alpha1) formed functional homooligomeric receptors that were fully resistant to bicuculline and were blocked by the specific GABA(C) antagonist (1,2,5, 6-tetrahydropyridine-4-yl)methylphosphinic acid and by zinc. Moreover, these chimeric receptors had a fast-desensitizing component, even faster than that of heterooligomeric GABA(A) receptors, in striking contrast to the almost nil desensitization of wild-type rho1 (wt rho1) receptors. To see whether the fast-desensitizing characteristic of the chimera was determined by the amino acids forming the ion channels, we replaced the second transmembrane segment (TM2) of rho1 by that of the alpha1-subunit of GABA(A). Although the alpha1-subunit forms fast-desensitizing receptors when coexpressed with other GABA(A) subunits, the sole transfer of the alpha1TM2 segment to rho1 was not sufficient to form desensitizing receptors. All this suggests that the slow-desensitizing trait of rho1 receptors is determined by a combination of several interacting domains along the molecule. [1] The inhibitory neurotransmitter, GABA, activates a variety of receptors in all areas of the CNS. Two major subtypes of GABA receptors are well known: (1) GABAA receptors are ligand-gated Cl- channels that consist of a heteromeric mixture of protein subunits forming a pentameric structure, and (2) GABAB receptors couple to Ca2+ and K+ channels via G proteins and second messengers. Here, Graham Johnston discusses evidence for a third major subclass of GABA receptors. GABAC receptors appear to be relatively simple ligand-gated Cl- channels with a distinctive pharmacology, in that they are not blocked by bicuculline and not modulated by barbiturates, benzodiazepines or neuroactive steroids. Compared with GABAA receptors, GABAC receptors are activated at lower concentrations of GABA and are less liable to desensitization. In addition, their channels open for a longer time. The pharmacology of these novel subtypes of GABA receptors may yield important therapeutic agents.[2] |
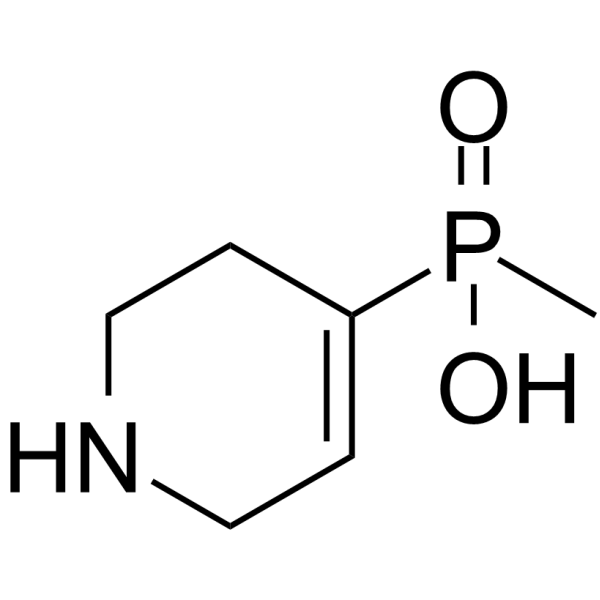
| 分子式 |
C6H12NO2P
|
|---|---|
| 分子量 |
161.13878
|
| 精确质量 |
161.061
|
| CAS号 |
182485-36-5
|
| PubChem CID |
5521
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
1.092
|
| tPSA |
59.14
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
10
|
| 分子复杂度/Complexity |
200
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CP(C1=CCNCC1)(=O)O
|
| InChi Key |
MFUKVPOVVKKLRQ-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C6H12NO2P/c1-10(8,9)6-2-4-7-5-3-6/h2,7H,3-5H2,1H3,(H,8,9)
|
| 化学名 |
methyl(1,2,3,6-tetrahydropyridin-4-yl)phosphinic acid
|
| 别名 |
TPMPA; 182485-36-5; methyl(1,2,3,6-tetrahydropyridin-4-yl)phosphinic acid; Tpmpa [MI]; Phosphinic acid, P-methyl-P-(1,2,3,6-tetrahydro-4-pyridinyl)-; UNII-TR7I0800L2; TR7I0800L2; CHEMBL397209;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 6.2058 mL | 31.0289 mL | 62.0578 mL | |
| 5 mM | 1.2412 mL | 6.2058 mL | 12.4116 mL | |
| 10 mM | 0.6206 mL | 3.1029 mL | 6.2058 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。