| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| Other Sizes |
|
| 靶点 |
IC50: angiotensin converting enzyme[1]
|
|---|---|
| 体外研究 (In Vitro) |
斯匹普利是一种非巯基血管紧张素转换酶(ACE)抑制剂前药,口服后可转化为活性代谢物斯匹普利特,主要用于治疗高血压。
|
| 体内研究 (In Vivo) |
在 TGM123 小鼠中,螺普利(喂食针;10 毫克/公斤;3 周)可减少饮酒量;在 TLM 小鼠中则不然。在接受螺旋西林治疗的小鼠脑膜中,ACE 活性降低了 40.2%。在实验中,它降低了转基因效应并通过了血脑屏障。 [2]
在 TGM123 小鼠中,但在 TLM 小鼠中,螺普利(注射用;10 mg/kg;3 周)可减少饮酒量 [2]。在接受治疗的小鼠中,吡罗普利使脑膜 ACE 活性降低了 40.2% [2]。螺普利能够穿透血脑屏障并在测试中防止转基因效应[2]。在自发性高血压大鼠中,螺普利可以抑制左心室肥厚,减轻心肌损伤,并刺激血管生成[3]。 药效学性质[1] 螺旋普利是一种前药,当代谢成活性的二酸形式螺旋普利时,对血管紧张素转换酶(ACE)具有有效的活性。在许多动物模型中,通过直接测量ACE活性或通过衰减血管紧张素i诱导的升压反应,证明了螺旋普利对血浆ACE活性的抑制作用。在高血压患者中,口服螺旋普利可产生短期(1或4小时)血浆ACE活性抑制75%至≥90%,而长期评估(长达6个月)可产生33%至86%的ACE活性降低。 在许多涉及高血压患者的临床试验中,使用螺旋普利可显著降低血压(见治疗疗效部分)。此外,在高血压或充血性心力衰竭患者中,螺旋普利可降低血管阻力。据报道,螺旋普利在人类中与左心室肥厚相关的一些结构参数(后壁厚度、左室质量和室间隔壁厚度)降低约8 ~ 17%,证实了动物模型的有利结果。有限的结果表明,螺旋普利通过显著减少左室后壁增厚来发挥其对左室肥厚的积极作用。 在志愿者或肾功能正常的患者中,螺旋普利似乎对肾小球滤过率或肾血流量没有明显的不良影响。然而,由于现有的关于该适应症的数据有限且相互矛盾,螺旋普利对肾功能损害患者的影响尚未得到充分的描述。 < 疗效[1] 在几项剂量发现研究中,每日一次的6 - 24mg剂量的螺旋普利在降低轻度至重度高血压患者的血压方面具有相似的疗效。在这些试验中,29 - 50%的患者血压恢复正常(治疗期结束时24小时给药后谷读数<90mm Hg),而研究终点收缩压谷和舒张压谷的平均降幅分别为10 - 18mm Hg和7 - 13mm Hg。每日一次的剂量小于6mg的斯匹普利通常效果低于高剂量,约12%的患者血压恢复正常,平均收缩压和舒张压分别降低约4 ~ 9mmhg和3 ~ 7mmhg。与15%和22%的安慰剂组患者的血压恢复正常相比,每日1次spirapril 6 - 24mg的患者血压恢复正常的比例为35% - 50%。 虽然与其他ACE抑制剂的直接比较数量有限,但几项比较螺旋普利与其他抗高血压药物的临床研究的结果是可用的。斯匹普利的降压效果与依那普利相似(18/17 vs 19/14mm Hg;n = 201)或卡托普利(10/10 vs 9/ 9mmhg;N = 169)。在213例接受8周治疗的中度至重度高血压患者中,Spirapril 12 - 24mg每日1次的血压正常率(37%)明显高于钙拮抗剂nitrendipine 20 - 40mg每日1次(24%)。 斯匹普利3 ~ 6mg每日1次可有效降低老年患者的高血压,在该适应症的单一比较研究中,其疗效与伊斯拉地平相似。个别报道称,糖尿病肾病患者的降压效果与伊地平相似,睡眠呼吸暂停患者的降压效果优于阿替洛尔、氢氯噻嗪和伊地平,这些都需要证实。 给药剂量[1] 临床试验数据表明,在高血压患者降压方面,每日口服一次剂量为6mg的螺旋普利与大剂量服用同样有效。在老年患者中,每日服用3 - 6mg的斯匹普利已显示出显著的降压效果。肾功能损害患者[肌酐清除率(CLCR) <80 ml/min]的结果表明,与大多数其他ACE抑制剂相比,在这种情况下不需要调整剂量。然而,鉴于缺乏关于其对肾功能影响的明确信息,严重肾功能衰竭(CLCR <30 ml/min)患者不应使用螺旋普利。 为了检测血管紧张素转换酶(ACE)抑制是否能预防自发性高血压大鼠(SHR)心肌损伤及对冠状动脉微血管的影响,我们对5周龄SHR幼鼠给予螺旋普利治疗3个月,并监测其血压(BP)变化。未处理的SHR作为对照。老鼠被杀死了;观察左心室形状、重量、壁厚,并对心室心肌进行形态计量学分析,以确定药物对心肌瘢痕形成的相对数量、单位面积心肌数量和平均病灶尺寸的影响。分析冠状动脉毛细血管的体积分数、表面、数值密度和氧气扩散距离。与对照组相比,处理过的SHR组的血压降低了20 - 30%,LV的重量和厚度分别降低了20%和21%。纤维化灶的数量和尺寸减少,导致心肌损伤量总体减少68%。最后,在处理过的SHR中,毛细血管剖面的数值密度增加28%,其横截面积减少13%,氧气从毛细血管壁到肌细胞的扩散距离减少14%。斯匹普利降低高血压SHR模型血压、左室重量和厚度,显著改善冠状动脉毛细血管微血管,减轻高血压心肌损害。这些结果可能归因于抑制血管紧张素II (All)的全身作用以及药物对可能在心肌内产生的All的局部保护作用。[3] |
| 动物实验 |
Animal/Disease Models: TGM123 mice (expressing a rat angiotensinogen transgene) and TLM (deficient the angiotensinogen gene) mice[2]
Doses: 10 mg/kg Route of Administration: Feeding needle ; 10 mg/kg; 3 weeks Experimental Results: Alter voluntary alcohol consumption in animals. Crossed the blood-brain barrier and may influence alcohol consumption mainly by decreasing central angiotensin II (AII) levels. After a 3-wk period of treatment with spinapril at 10 mg/kg of body weight, the animals were killed by decapitation. The brains were rapidly removed and stored until use at −80°C. Membranes were prepared according to the method of Hulme and Buckley and were stored until use at −80°C in a 50 mM Tris buffer containing 320 mM sucrose. ACE activity was measured via a modified fluorimetric method originally developed by Friedland and Silverstein. In brief, 10 μl of a membrane preparation was incubated for 30 min with 10 μl of 0.025 M hippuryl-histidyl-leucine as substrate in a chloride-containing phosphate buffer at pH 8.3. The reaction was stopped by addition of 1 ml of 0.4 M sodium hydroxide; fluorescence was developed by a reaction of the produced histidyl-leucine with 100 μl of 2% o-phthalaldehyde in methanolic solution. After acidification with 3 M hydrochloric acid and excitement at 365 nm, the fluorescence was measured at 500 nm. We used histidyl-leucine as a standard. Controls of specificity were carried out with 10−6 M of the specific ACE inhibitor lisinopril. The total protein contents of the membrane preparations were determined by a Bradford assay. Statistical calculations were done by applying a t test. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Bioavailability is 50% following oral administration. Metabolism / Metabolites Hepatic. Converted to spiraprilat following oral administration. Biological Half-Life 30 to 35 hours Pharmacokinetic Properties [1] Following oral administration, the mean bioavailability of spirapril is 50%. Conversion of spirapril to its active diacid metabolite occurs rapidly, with maximum plasma concentrations (Cmax) of spiraprilat reached 1.8 to 3.0 hours after an oral spirapril dose. The disposition of spiraprilat in plasma is biphasic, with an initial phase half-life of 1.5 to 2.2 hours. High affinity binding of spiraprilat to ACE is responsible for a typical terminal elimination half-life of ~t30 to 40 hours. Elimination of spiraprilat occurs by both renal and nonrenal (hepatic) mechanisms. Spiraprilat does not exhibit any clinically significant accumulation (as measured by trough plasma concentrations 24 hours postdose) in patients with renal failure and dosage adjustment is not required. The pharmacokinetics of spiraprilat are altered in the elderly [30% increase in area under the concentration-time curve (AUC) and Cmax] and in patients with liver disease (30% decrease in AUC). |
| 毒性/毒理 (Toxicokinetics/TK) |
Tolerability [1]
Spirapril has a tolerability profile which is broadly similar to that of other ACE inhibitors, the most common adverse events reported in dose-finding and comparative trials being dizziness (up to 10.7%), headache (up to 13.1%) and fatigue (1.8 to 6.0%) [pooled n = 736]. The rates of these events, and of others commonly reported with ACE inhibitors, were generally similar for spirapril and placebo. The incidence of cough with spirapril (0 to ~t4%) in the few studies which provided this information appeared to be lower than values reported for other members of this drug class (typical range 1 to 10%, with peak incidences of between 15 and 25%), although direct prospective comparisons with other ACE inhibitors are required to confirm this finding. First-dose hypotension has not been reported in studies of spirapril. In the few direct clinical comparisons published to date, rates for adverse events or patient withdrawals with spirapril were similar to those with captopril and lower than those with enalapril or nitrendipine. 5311447 rat LD50 oral >2500 mg/kg Toxicologist., 5(98), 1985 5311447 rat LD50 intraperitoneal 600 mg/kg Toxicologist., 5(98), 1985 5311447 mouse LD50 oral >2500 mg/kg Toxicologist., 5(98), 1985 5311447 mouse LD50 intraperitoneal 400 mg/kg Toxicologist., 5(98), 1985 |
| 参考文献 | |
| 其他信息 |
Spirapril Hydrochloride is the hydrochloride salt form of spirapril, a prodrug and non-sulfhydryl angiotensin converting enzyme (ACE) inhibitor with antihypertensive activity. Spirapril is converted in the body to its active metabolite spiraprilat. Spiraprilat competitively binds to and inhibits ACE, thereby blocking the conversion of angiotensin I to angiotensin II. This prevents the potent vasoconstrictive actions of angiotensin II and results in vasodilation. Spirapril also decreases angiotensin II-induced aldosterone secretion by the adrenal cortex, which leads to an increase in sodium excretion and subsequently increases water outflow.
See also: Spirapril (is salt form of). Spirapril is a dipeptide, a dithioketal, an azaspiro compound, a dicarboxylic acid monoester, an ethyl ester, a tertiary carboxamide, a secondary amino compound and a pyrrolidinecarboxylic acid. It has a role as a prodrug, an EC 3.4.15.1 (peptidyl-dipeptidase A) inhibitor and an antihypertensive agent. It is functionally related to a spiraprilat. Spirapril is an ACE inhibitor antihypertensive drug used to treat hypertension. Spirapril is converted to the active spiraprilat after administration. ACE inhibitors are used primarily in treatment of hypertension and congestive heart failure. Spirapril is a prodrug and non-sulfhydryl angiotensin converting enzyme (ACE) inhibitor with antihypertensive activity. Spirapril is converted in the body to its active metabolite spiraprilat. Spiraprilat competitively binds to and inhibits ACE, thereby blocking the conversion of angiotensin I to angiotensin II. This prevents the potent vasoconstrictive actions of angiotensin II and results in vasodilation. Spiraprilat also decreases angiotensin II-induced aldosterone secretion by the adrenal cortex, which leads to an increase in sodium excretion and subsequently increases water outflow. Drug Indication Spirapril is an ACE inhibitor class drug used to treat hypertension. Mechanism of Action Spiraprilat, the active metabolite of spirapril, competes with angiotensin I for binding at the angiotensin-converting enzyme, blocking the conversion of angiotensin I to angiotensin II. Inhibition of ACE results in decreased plasma angiotensin II. As angiotensin II is a vasoconstrictor and a negative-feedback mediator for renin activity, lower concentrations result in a decrease in blood pressure and stimulation of baroreceptor reflex mechanisms, which leads to decreased vasopressor activity and to decreased aldosterone secretion. Spiraprilat may also act on kininase II, an enzyme identical to ACE that degrades the vasodilator bradykinin. Pharmacodynamics Spirapril is an angiotensin-converting enzyme (ACE) inhibitor. ACE is a peptidyl dipeptidase that catalyzes the conversion of angiotensin I to the vasoconstrictor substance, angiotensin II. By blocking ACE, spirapril decreases angiotensin II which is a vasoconstrictor and inducer of aldosterone. So by inhibiting the enzymes, aldosterone secreation is decreased (so less sodium is reabsorbed) and there is a decrease in vasoconstriction. Combined, this leades to a decrease in blood pressure. Spirapril is a non-sulfhydryl angiotensin converting enzyme (ACE) inhibitor prodrug which is converted to the active metabolite spiraprilat following oral administration, and which has been evaluated primarily for the treatment of hypertension. In dose-finding studies of patients with mild to severe hypertension, spirapril > or = 6 mg once daily produced reductions in blood pressure of approximately 10 to 18 mm Hg (systolic) and 7 to 13 mm Hg (diastolic) [24-hour postdose trough readings at the end of the treatment period]. Blood pressure normalisation (trough diastolic blood pressure < or = 90 mm Hg) had occurred in 29 to 50% of patients at the end of these investigations. The dose-response curve for spirapril appears to be flat for doses of 6 to 24 mg once daily. Comparisons with other ACE inhibitors are limited in number, and further studies are required before the relative antihypertensive efficacy of spirapril can be fully evaluated. However, in single, well controlled clinical trials, spirapril produced similar reductions in blood pressure to those seen with enalapril or captopril. When given as monotherapy or in combination with hydrochlorothiazide, spirapril may offer potential advantages over the calcium antagonist nitrendipine. Spirapril is generally well tolerated and produces an adverse event profile similar to that of other ACE inhibitors. Data from small studies suggest that spirapril can be used without dosage adjustment in patients with renal impairment, as a consequence of its dual renal and hepatic clearance mechanisms. This is in contrast to most ACE inhibitors, which are eliminated by a predominantly renal mechanism that results in accumulation of the active metabolite when renal function is impaired. However, the utility of spirapril in this patient group has yet to be fully determined because of conflicting data regarding its effects on renal function. Thus, spirapril is an effective antihypertensive agent which is well tolerated. Further comparative trials are needed to fully determine its efficacy with respect to other ACE inhibitors, and a better understanding of its effects on renal function will clarify its role in hypertensive patients with renal failure. [1] |
| 分子式 |
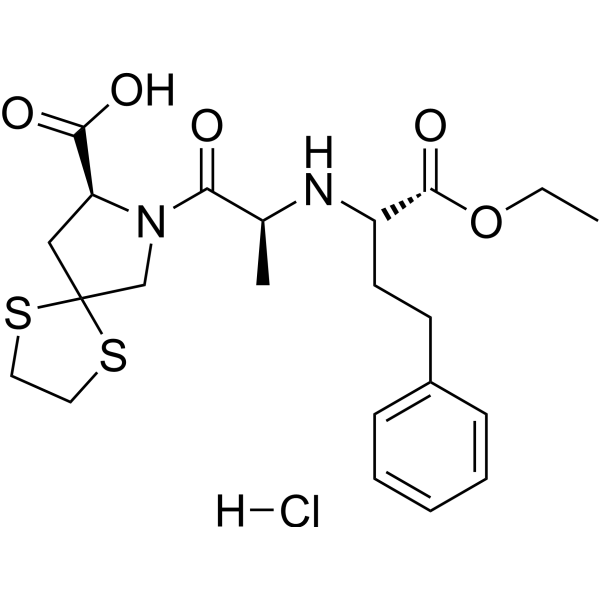
C22H31CLN2O5S2
|
|---|---|
| 分子量 |
503.07
|
| 精确质量 |
502.136
|
| 元素分析 |
C, 52.53; H, 6.21; Cl, 7.05; N, 5.57; O, 15.90; S, 12.75
|
| CAS号 |
94841-17-5
|
| 相关CAS号 |
Spirapril;83647-97-6; Spirapril hydrochloride;94841-17-5; 200872-06-6 (HCl hydrate)
|
| PubChem CID |
6850814
|
| 外观&性状 |
White to off-white solid powder
|
| 沸点 |
697.8ºC at 760mmHg
|
| 熔点 |
192-194ºC (dec.)
|
| 闪点 |
375.8ºC
|
| LogP |
3.521
|
| tPSA |
146.54
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
10
|
| 重原子数目 |
32
|
| 分子复杂度/Complexity |
650
|
| 定义原子立体中心数目 |
3
|
| SMILES |
CCOC(=O)[C@H](CCC1=CC=CC=C1)N[C@@H](C)C(=O)N2CC3(C[C@H]2C(=O)O)SCCS3.Cl
|
| InChi Key |
CLDOLNORSLLQDI-OOAIBONUSA-N
|
| InChi Code |
InChI=1S/C22H30N2O5S2.ClH/c1-3-29-21(28)17(10-9-16-7-5-4-6-8-16)23-15(2)19(25)24-14-22(30-11-12-31-22)13-18(24)20(26)27;/h4-8,15,17-18,23H,3,9-14H2,1-2H3,(H,26,27);1H/t15-,17-,18-;/m0./s1
|
| 化学名 |
(8S)-7-[(2S)-2-[[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]propanoyl]-1,4-dithia-7-azaspiro[4.4]nonane-8-carboxylic acid;hydrochloride
|
| 别名 |
Spirapril hydrochloride; 94841-17-5; Spirapril HCl; Spirapril hydrochloride [USAN]; Sch 33844;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 100 mg/mL (198.78 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9878 mL | 9.9390 mL | 19.8779 mL | |
| 5 mM | 0.3976 mL | 1.9878 mL | 3.9756 mL | |
| 10 mM | 0.1988 mL | 0.9939 mL | 1.9878 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。