| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| Other Sizes |
| 靶点 |
RAS family: KRAS(G12C); c-Raf; H-Ras; NRAS rG4
|
|---|---|
| 体外研究 (In Vitro) |
RMC-7977是一种可逆的三复合RAS抑制剂,对突变型和野生型KRAS、NRAS和HRAS变体(RAS(ON)多选择性抑制剂)的活性状态具有广谱活性。临床前,RMC-777对携带各种RAS基因型的RAS-附加肿瘤表现出有效的活性,特别是对具有KRAS密码子12突变的癌症模型(KRASG12X)。[1]
RMC-7977抑制了FLT3-ITD(Molm-14,MV4-11)、KITN822K(Kasumi-1,SKNO-1)和RAS突变(NRASQ61L-OCIAML-3,HL-60,KRASG13D-NOO-1)驱动的AML细胞系的细胞增殖,IC 50值在5至33 nM之间,并抑制了MAPK途径MEK、ERK和RSK下游效应器的磷酸化。我们还评估了RMC-797在长期暴露于FLT3i后发生继发性NRASG12C或NRASQ617K突变的耐药Molm-14细胞中的活性。在低至5 nM的浓度下,RMC-7977在两种NRAS突变抗性细胞系中恢复了对吉尔特替尼的敏感性。使用胱天蛋白酶3/7测定,我们观察到RMC-7977诱导了AML细胞系的凋亡,但程度不同。我们之前已经证明,有效的MAPK信号抑制会增加对BCL2的凋亡依赖性。因此,在RMC-7977中添加venetoclax显著增强了FLT3、KIT和NRAS突变细胞系中胱天蛋白酶的激活。在细胞活力测定中,RMC-7977和venetoclax显示出高协同活性,如Bliss独立模型所评估的。[2] 鉴于患者体内肿瘤异质性与靶向治疗的临床耐药性有关,研究人员在模型中研究了RMC-7977的体外活性,这些模型再现了FLT3i治疗患者中观察到的克隆生长模式。我们将荧光标记的细胞系与FLT3-ITD(Molm-14)、FLT3-ITD和NRAS共突变(Molm-14-NRASQ61K)和仅NRAS突变(OCIAML-3)混合,用RMC-7977单独和联合(与吉尔特替尼或venetoclax)处理混合物96小时,然后通过流式细胞术评估细胞存活率。Gilteritinib和Gilteritini/venetoclax组合被选择用于携带NRAS突变的细胞的存活,但RMC-7977抑制了所有细胞群的生长。RMC-7977和吉尔特替尼的组合在含有FLT3和FLT3-NRAS共突变的混合物中具有优异的活性,但在单独携带NRAS突变的细胞中,与RMC-7997单一疗法相比没有额外的益处。令人惊讶的是,RMC-7977和venetoclax的组合在所有细胞系模型中都能有效地抑制细胞存活率,并且明显优于单独使用RMC-7997。正在进行体内研究,调查RMC-7977和RMC-7997组合在RAS突变体/FLT3i抗性患者衍生的异种移植物模型中的耐受性和活性,并将进行介绍。[2] |
| 体内研究 (In Vivo) |
RMC-7977治疗导致肿瘤消退,并且在不同的RAS-添加的临床前癌症模型中具有良好的耐受性。此外,RMC-7977抑制了KRASG12C癌症模型的生长,这些模型由于RAS途径信号的恢复而对KRAS(G12C)抑制剂具有耐药性。因此,RAS(ON)多选择性抑制剂可以靶向多种致癌和野生型RAS亚型,并有可能治疗各种RAS成瘾的癌症,这些癌症具有高度未满足的临床需求。一种相关的RAS(ON)多选择性抑制剂RMC-6236目前正在KRAS突变实体瘤患者中进行临床评估(ClinicalTrials.gov标识符:NCT05379985)。[1]
在这项研究中,研究人员评估了RMC-7977在各种PDAC模型中的治疗潜力。我们观察到,在体内耐受良好的暴露条件下,直接抑制RAS后,模型具有广泛而明显的抗肿瘤活性。药理学分析显示,肿瘤组织与正常组织对RMC-7977的反应不同。治疗后的肿瘤表现出凋亡波和持续的增殖停滞,而正常组织的增殖仅短暂下降,没有凋亡的证据。在本地KPC小鼠模型中,RMC-7977治疗导致生存期显著延长,随后治疗复发。对复发肿瘤的分析表明,Myc拷贝数增加是一种普遍的候选耐药机制,可以通过体外组合TEAD抑制来克服。这些数据共同为在PDAC环境中使用广谱RAS-GTP抑制建立了强有力的临床前理论基础,并确定了一种有前景的候选联合治疗方案来克服单一疗法的耐药性[3]。 |
| 酶活实验 |
RAS–RAF和RAS–CYPA TR-FRET[1]
如前所述,使用时间分辨荧光共振能量转移(TR-FRET)来评估野生型RAS或突变致癌RAS蛋白与BRAF的RAS结合结构域之间相互作用的破坏,并评估RAS蛋白与CYPA12之间相互反应的诱导。 CYPA结合亲和力[1] 如前所述,化合物对CYPA(Kd1)的结合亲和力通过Biacore 8K仪器上的SPR进行评估12。 RAS结合亲和力[1] 在Biacore 8K仪器上通过SPR评估化合物结合的CYPA对上述突变致癌RAS蛋白(Kd2)的结合亲和力。AviTag RAS[残基1-169]固定在链霉抗生物素蛋白传感器芯片上,并在测定缓冲液(10 mM HEPES NaOH pH 7.4,150 mM NaCl,0.005%v/v表面活性剂P20,2%v/v DMSO,25μM CYPA)中使不同浓度的化合物流过芯片。使用稳态亲和力模型或1:1结合(动力学)模型拟合SPR传感图,以评估RAS结合的解离常数(Kd)。 |
| 细胞实验 |
细胞RAS–RAF和RAS–CYPA测定[1]
将U2OS细胞或具有PPIA基因敲除的U2OS细胞以每孔500000个细胞的速度接种在6孔板中,并孵育过夜。将含有指定突变的KRAS4B或其他小GTP酶克隆到pNLF-N或pHTN质粒中,分别用N端纳米萤光素酶或HaloTag融合物表达。将全长CYPA克隆到pHTN中,将RAF1的RBD(残基51-149)克隆到pHTC中,将全长RALGDS克隆到pHTC中,将PIK3CA克隆到pNLF-N中,将SOS1的催化结构域(残基558-1049)克隆到pNL F-N中。按照制造商的方案,使用Fugene HD试剂将KRAS和效应质粒转染U2OS细胞,将小GTP酶和CYPA质粒转染U2OSPPIA-KO细胞。第二天,用胰蛋白酶收集细胞,并在含有4%FBS和1:1000稀释的NanoBRET 618 HaloTag配体的OptiMem无酚红培养基(Gibco)中的白色组织培养处理的96孔板中重新接种。对于终点浓度响应曲线,在含有4%FBS的OptiMem无酚红培养基中,将间日嗪纳米萤光素酶底物添加到1倍浓度。在Perkin-Elmer Envision平板读数器上测量纳米BRET信号之前,添加不同浓度的抑制剂并孵育1或4小时。对于动力学分析,使用去哌嗪纳米萤光素酶底物代替间日嗪,并将平板放置在预平衡至37°C和5%CO2的Cytation5多模式阅读器中。平衡1小时后,加入RMC-7977(50 nM)并测量纳米BRET信号。 PRISM分析[1] RMC-7977在博德研究所建立的931个PRISM DNA条形码细胞系中进行了筛选。简而言之,每个池中有20-25个细胞系被放置在384孔板中,并用RMC-7977以8个剂量以3倍稀释,从10µM开始处理5天。然后在TCL mRNA裂解缓冲液中裂解细胞,然后进行逆转录PCR。然后如前所述进行条形码检测和单变量和多变量分析43。数据分析见补充方法。我们分析的最新代码位于github链接:https://github.com/cmap/dockerized_mts. View More
Cell panel[1] 细胞增殖分析[1] 将细胞接种在2D的384孔或96孔组织培养处理板中,并孵育过夜。或者,将细胞接种在圆底超低附着96孔板中,以1000rpm离心10分钟以沉淀细胞,并孵育过夜或长达72小时以形成3D球体。将细胞暴露于化合物或DMSO对照(0.1%v/v)的连续稀释液中120小时。根据制造商的方案,通过CellTiter Glo 2.0试剂(2D CTG)(Promega,G9243)或3D CellTiter Glo试剂(3D CTG)测定细胞存活率。使用Perkin-Elmer Enspire的SpectraMax M5平板阅读器检测发光。将发光信号归一化为载体处理的孔(归一化信号(%)=(发光(处理)/平均发光(载体))×100%)。对于PSN1和HUPT3,原始信号被归一化为车辆控制和低信号控制化合物((样本信号-平均低控制信号)/(平均车辆信号-平均高控制信号)×100%)。对于用RMC-7977和桑格列菲林A竞争性CYPA抑制剂(3 mM)组合处理的NCI-H441和AsPC-1细胞,发光信号被标准化为仅用CYPA抑制剂处理的对照的发光信号(标准化信号(%)=(发光(处理)/平均发光(仅限CYPA抑制剂)×100%)。 细胞和上清液的生物分析[1] 1000万个细胞在37°C下暴露于1×106个细胞ml−1的悬浮液中的RMC-7977(10、100或1000 nM)1小时。通过离心将细胞制成颗粒,保留1ml上清液并在-80°C下冷冻。细胞颗粒在冷PBS中洗涤两次,在干冰和乙醇的浆液中快速冷冻之前,称量含有细胞颗粒的预先称重的试管。通过液相色谱-串联质谱(LC-MS/MS)方法测定细胞沉淀和上清液中RMC-7977的浓度。将细胞沉淀样品重新悬浮在细胞培养基中(根据需要稀释),然后作为上清液处理。用3倍体积的含有内标特非那定(2.5 ng ml−1)的乙腈淬灭上清液或再悬浮细胞(50µL)的等分试样。样品被涡旋、离心,并在配备岛津AD LC系统的Sciex 6500+三重四极杆质谱仪上进行分析。采用Waters ACQUITY UPLC BEH C4 1.7µm(2.1×50 mm)柱,梯度洗脱进行化合物分离。使用多重反应监测通过正电喷雾电离检测RMC-7977和内标(RMC-7977:m/z 865.273/833.500;特非那定:m/z 471.939/436.300)。定量下限为0.25 ng ml-1,校准范围为0.25至400 ng ml-1。使用每个细胞颗粒的质量(减去空管的质量)和已知的细胞数量计算RMC-7977的细胞内浓度,假设细胞的体积约为2000µm3,细胞的密度约为水的密度(因此,细胞体积=细胞质量);并且CYPA-KO细胞中任何超过培养基浓度的化合物都可能是膜结合的。对于每种测试的RMC-7977浓度,确定细胞颗粒中的化合物浓度与培养基中的化合物的比率。 小鼠细胞活力测定[3] 将具有KrasG12C或KrasG12D突变的PDAC小鼠细胞系(初始治疗或来源于RMC-7977治疗的终点KPC肿瘤)以2×103接种在96孔板中。24小时后,用DMSO或RMC-7977、ERK抑制剂(SCH772984)或MEK抑制剂(曲美替尼)的系列稀释液处理细胞。72小时后,根据制造商的说明,通过使用CellTiter Glo发光细胞活力测定法测量ATP水平来评估细胞活力。或者(在比较幼稚和抗性细胞系的实验中),使用钙黄绿素AM对活细胞进行荧光标记(在500 nMr下孵育20分钟),并使用SpectraMax i3X多模式检测平台进行计数。每个生物复制品都进行了三次技术复制,每个细胞系总共进行了3-4次生物复制。通过将药物处理值标准化为DMSO对照来计算生长百分比,DMSO对照设置为100%。从GraphPad Prism中的生物复制中生成了四参数药物反应曲线。绘制了每种测试稀释液的平均值±标准差。 对于RMC-7977处理过的幼稚和抗性细胞系的协同评估测试,使用了类似的方案,但进行了以下更改:细胞系接种后24小时,使用D300e数字分配器将RMC-7997、IAG933(2714434-21-4)或联合处理添加到细胞中。使用R Studio中的SynergyFinder软件包,使用Excess over Bliss方法计算每个细胞系的平均协同值。 人细胞系增殖试验[3] 作为Crown Bioscience筛选的各种组织类型的人类癌症细胞系的一部分,测试了19个PDAC细胞系对RMC-7977的敏感性。这些PDAC细胞系携带KRASG12D、KRASG12V、KRASG22C、KRASQ61H和BRAFV487_P492delinsA突变。为了测量细胞增殖的抑制作用,将细胞在甲基纤维素中培养,并用由帝肯D300e数字分配器分配的RMC-7977(最高浓度为1µM)或DMSO的连续稀释液处理三次。在使用CellTiter Glo测量ATP水平之前,将细胞孵育120小时。每个生物复制品都进行了三次技术复制,每个细胞系共进行了3-4次生物复制。通过将药物处理值标准化为DMSO对照来计算生长百分比,DMSO对照设置为100%。将标准化CTG测定读数绘制为对数摩尔抑制剂浓度的函数,并将四参数S形浓度-反应模型拟合到数据中。绘制了每种测试稀释液的平均值±标准差。 将携带野生型KRAS或KRASQ61H的PDAC细胞系以每孔500-4000个细胞的速度接种在透明平底96孔板上,并在使用D300e数字分配器添加指定浓度的RMC-7977或DMSO之前生长24小时。处理后,将细胞再孵育3-5天,之后使用Calcein AM对活细胞进行荧光标记(在500 nM下孵育20分钟),并使用SpectraMax i3X多模式检测平台进行计数。实验在第0天使用独立的培养板进行标准化。通过将药物处理值标准化为DMSO对照来计算生长百分比,DMSO对照设置为100%。将四参数S型浓度-反应模型拟合到至少三个生物重复的数据中。绘制了每种测试稀释液的平均值±标准差。 为了评估RMC-7977和IAG933的协同作用,使用了类似的方案,但有以下变化:细胞系接种后24小时,RMC-7997、IAG933(2714434-21-4),或使用D300e数字分配器(帝肯)将组合添加到细胞中。每个细胞系被视为一个单独的生物复制品(n=8)。使用R(Studio)中的SynergyFinder软件包,使用超额幸福法计算每个细胞系的平均协同值。 蛋白质印迹分析[3] 在生长培养基中,以每孔7.5×103至4×106个细胞的速度将细胞接种在6孔板或100毫米培养皿中。孵育过夜后,加入指定化合物(RMC-7977、IAG933或DMSO(0.1%v/v))并孵育指定时间点。用冰冷的PBS洗涤细胞两次,并用NP-40裂解缓冲液(赛默飞世尔,J60766)、MSD-Tris溶血缓冲液(MSD,R60TX-2)、RIPA缓冲液(50 mM Tris-HCl,pH 7.5,150 mM NaCl,1%NP-40,0.5%脱氧胆酸钠,0.1%SDS)或含有1%Triton X-100、20 mM Tris-HCl、150 mM NaCl和1 mM EDTA的裂解缓冲液裂解细胞。所有裂解缓冲液都补充了蛋白酶和磷酸酶抑制剂。在4°C下以21000g离心10分钟之前,刮取并收集裂解物。通过BCA测定定量含蛋白质的上清液,并在95°C下用LDS和还原剂变性等量的蛋白质。样品在12%或4-12%的Bis-Tris聚丙烯酰胺凝胶上分离,然后使用iBlot 2.0系统或湿转移转移转移到硝化纤维或PVDF膜上。在4°C下用第一抗体探测过夜之前,将膜在Intercept TBS缓冲液(Li-Cor,927-60001)或3-5%的牛奶中封闭。根据需要添加二抗,并在Li-Cor Odyssey成像仪上对膜进行成像。或者,将膜与HRP连接的二抗一起孵育,并使用ChemiDoc XRS+或ChemiDoc MP成像仪用Clarity或ClarityMax化学发光底物进行显影。 |
| 动物实验 |
RMC-7977 formulation[3]
For in vitro studies RMC-7977 was re-suspended in DMSO (Fisher Bioreagents, BP231-100) and used at 10 mM stock concentration. For use in the in vivo studies RMC-7977 was prepared using the formulation made of 10/20/10/60 (%v/v/v/v) DMSO/PEG 400/Solutol HS15/water. The same vehicle formulation was used for all control groups. In vivo xenograft studies[3] RMC-7977 treatment Tumour-bearing mice were randomized and assigned into groups (n = 3–10 per group). Vehicle or RMC-7977 was administered via oral gavage daily at 10 mg kg−1 and mice were treated for 21–28 days. Studies were terminated early if tumour burden reached humane endpoint. Body weights were collected twice a week during the study. Means ± s.e.m were plotted in the waterfall plots. For the single-dose pharmacokinetic–pharmacodynamic study, mice were randomized and assigned into groups (n = 3–6 per dose and timepoint). A single dose of RMC-7977 was administered orally at 10 mg kg−1, 25 mg kg−1 or 50 mg kg−1. Tissues (including tumour, colon and skin) were collected at indicated timepoints and either fixed in 10% formalin, embedded in Optimal Cutting Temperature (OCT; Sakura, 4583) solution or snap-frozen in liquid nitrogen for further analysis. Whole blood was transferred into K2EDTA Microtainer tubes (BD, 365974), incubated for 5 min and snap-frozen in liquid nitrogen. In vivo allograft studies[3] Mouse studies: All mouse allograft studies and procedures related to animal handling, care and treatment were conducted in compliance with all applicable regulations and guidelines of the Institutional Animal Care and Use Committee (IACUC). Female C57BL/6J (strain 000664) mice aged 6–8 weeks from the Jackson Laboratory were used for these studies. Generation of allograft models In order to generate subcutaneous allograft tumours, each mouse was inoculated in the right flank with 3 × 105 of KPCY 6499c4 tumour cells in 0.1 ml of Matrigel:PBS (1:1). Treatments were started when the average tumour size reached 140 mm3. Tumour size was measured at two dimensions using a digital calliper, and the tumour volume in mm3 was calculated using the formula volume = (width2 × length)/2. Mice on studies were weighed and tumours were measured 2 times a week. To generate orthotopic allograft tumours, 5 × 104 KPCY 6499c4 tumour cells in 20 µl PBS/Matrigel mixtures (1:1) were implanted directly into the mouse pancreas through a laparoscopic incision. Treatments were started when the average tumour size reached ~50 mm3. Body weights were measured and tumour growth was monitored by ultrasound twice weekly. RMC-7977 treatment: Tumour-bearing mice were randomized, assigned into groups (n = 9–10 per group), and treated daily via oral gavage with vehicle or RMC-7977 (10 mg kg−1). For subcutaneous KPCY study, survival endpoint was defined as: tumour volume reaching 2000 mm3 or mice showing any clinical signs, including severe ulceration. For orthotopic KPCY study, survival endpoint was defined as (1) mice showing any clinical signs including hunching or fluid in the abdomen, or (2) tumour dimensions exceeding the imaging frame of the ultrasound. Body weights were measured twice a week during the study. Tissue was collected either at 4 h or 24 h after last dose and preserved as previously described (see ‘In vivo xenograft studies’). View More
In vivo GEMM studies[3] Pharmacodynamic study in KPC mice [3] Tumour formation in KPC mice was monitored by bi-weekly palpation. Upon detection of a 4–7 mm diameter tumour by ultrasound, KPC mice were randomized and treated with vehicle (n = 6) or RMC-7977 (50 mg kg−1; n = 11). Treatments were performed every other day via oral gavage for 1 week. Mouse health status and weight were checked daily and ultrasounds (Vevo 3100) were performed every third day to monitor tumour growth. Following two consecutive ultrasounds, RMC-7977-treated mice were euthanized either 4 (n = 7) or 24 h (n = 4) after last dose and vehicle-treated mice were euthanized between 4–24 h post last dose. Tissue was collected and preserved as previously described (see ‘In vivo xenograft studies’). Additional group of KPC mice was also treated with a single dose of RMC-7977 (n = 10) or vehicle (n = 3) and tissues were collected at 4 or 24 h post-dose as previously described. Pharmacodynamic study in KPCY mice [3] KPCY mice were enroled upon detection of a 15–100 mm3 tumour measured via ultrasound. Mice were randomized into groups and treated with vehicle (n = 6) or RMC-7977 (25 mg kg−1; n = 8). Treatments were performed every day via oral gavage for 15 days and ultrasounds were performed on day 8 and 15. Mice were euthanized after last dose and tissue was collected and preserved as previously described (see ‘In vivo xenograft studies’). Survival study in KPC mice [3] For survival study, KPC mice with 4–7 mm diameter tumours (as measured by ultrasound) were enroled and treated every other day with vehicle (n = 9) or RMC-7977 (50 mg kg−1; n = 13). Mouse health status and weight were checked daily and ultrasounds were performed every third day to monitor tumour growth. The survival endpoint was determined by overall health criteria scoring, where endpoint is determined by a score of 5 or greater based on the following criteria: moribund, immediate euthanasia; abdominal distention due to haemorrhagic ascites, 5 pts; mild difficulty beathing, 5 points; hypothermia, 5 points; abdominal distention due to chylous ascites, 3 points; loss of over 20% enrolment body weight, 3 points; failure of grasp test, 3 points; jaundice or pallor, 3 points; weak grasp test, 2 points; failure to interact with other mice, 1 point; hunched, 1 point; pilorection/failure to groom, 1 point. Mouse blood and tumour sample bioanalysis[1] Whole-blood and tumour concentrations of RMC-7977 were determined using LC–MS/MS methods. Tumour tissue samples were homogenized with a 10× volume of methanol/15 mM PBS (1:2, v:v). Sample preparation and analysis on a Sciex 6500+ triple quadrupole mass spectrometer equipped with an ACQUITY UPLC system were performed as previously described12. RMC-7977 and internal standard verapamil were detected by positive electrospray ionization using multiple reaction monitoring (RMC-7977: m/z 865.4/706.4; verapamil: m/z 455.2/164.9). |
| 参考文献 | |
| 其他信息 |
RAS oncogenes (collectively NRAS, HRAS and especially KRAS) are among the most frequently mutated genes in cancer, with common driver mutations occurring at codons 12, 13 and 611. Small molecule inhibitors of the KRAS(G12C) oncoprotein have demonstrated clinical efficacy in patients with multiple cancer types and have led to regulatory approvals for the treatment of non-small cell lung cancer2,3. Nevertheless, KRASG12C mutations account for only around 15% of KRAS-mutated cancers4,5, and there are no approved KRAS inhibitors for the majority of patients with tumours containing other common KRAS mutations. Here we describe RMC-7977, a reversible, tri-complex RAS inhibitor with broad-spectrum activity for the active state of both mutant and wild-type KRAS, NRAS and HRAS variants (a RAS(ON) multi-selective inhibitor). Preclinically, RMC-7977 demonstrated potent activity against RAS-addicted tumours carrying various RAS genotypes, particularly against cancer models with KRAS codon 12 mutations (KRASG12X). Treatment with RMC-7977 led to tumour regression and was well tolerated in diverse RAS-addicted preclinical cancer models. Additionally, RMC-7977 inhibited the growth of KRASG12C cancer models that are resistant to KRAS(G12C) inhibitors owing to restoration of RAS pathway signalling. Thus, RAS(ON) multi-selective inhibitors can target multiple oncogenic and wild-type RAS isoforms and have the potential to treat a wide range of RAS-addicted cancers with high unmet clinical need. A related RAS(ON) multi-selective inhibitor, RMC-6236, is currently under clinical evaluation in patients with KRAS-mutant solid tumours (ClinicalTrials.gov identifier: NCT05379985).[1]
FLT3 inhibitors (FLT3i) such as gilteritinib are clinically active in AML, but their use is limited by resistance due to emergence of clones with RAS/MAPK mutations, both alone and in combination with FLT3 mutations, or with other drivers such as KIT mutations. RAS mutations are also commonly associated with relapse on IDH1/2 inhibitors and the BCL2 inhibitor venetoclax. Importantly, multiple heterogeneous resistant clones are identified at the time of relapse. In addition, 5-10% of all de novo patients harbor oncogenic RAS mutations. Patients with RAS mutations or other mutations that activate RAS/MAPK signaling do not benefit from clinically approved targeted therapies. Targeting oncogenic RAS has been historically challenging and inhibition of downstream effectors of the MAPK pathway, such as MEK, demonstrated modest activity and high toxicity in clinical trials. Effective inhibition of the RAS/MAPK pathway is therefore a critical unmet need in AML. RMC-7977 is a potent, oral small molecule inhibitor of both wild-type and mutant GTP-bound RAS oncoproteins (RAS MULTI) and is a preclinical tool compound representative of the clinical candidate RMC-6236, currently in clinical evaluation (NCT05379985). RMC-7977 non-covalently binds to the intracellular chaperone cyclophilin A, generating a neomorphic interface with high affinity for all isoforms of RAS. The resulting tri-complexes sterically block RAS-effector interactions required for propagating oncogenic signals. We report in vitro data supporting preclinical utility of RAS MULTI(ON) inhibition in AML models harboring RAS mutations, including those with resistance to FLT3i due to hyperactive RAS signaling.Given that intrapatient tumor heterogeneity is associated with clinical resistance to targeted therapies, we investigated the in vitro activity of RMC-7977 in models that recapitulate patterns of clonal outgrowth observed in patients treated with FLT3i. We mixed fluorescently-tagged cell lines with FLT3-ITD (Molm-14), FLT3-ITD and NRAS co-mutations (Molm-14 NRASQ61K) and NRAS-only mutations (OCIAML-3), treated the mixtures with RMC-7977 alone and in combination (with gilteritinib or venetoclax) for 96 hours, then assessed cell viability via flow cytometry. Gilteritinib and the gilteritinib/venetoclax combination selected for survival of cells harboring NRAS mutations, but RMC-7977 inhibited outgrowth of all cell populations. The combination of RMC-7977 and gilteritinib had superior activity in mixtures containing FLT3 and FLT3- NRAS co-mutations, but no additional benefit over RMC-7977 monotherapy in cells harboring NRAS mutations alone. Strikingly, the combination of RMC-7977 and venetoclax potently suppressed cell viability equally in all cell line models and was significantly superior to RMC-7977 alone. In vivo studies investigating the tolerability and activity of RMC-7977 and RMC-7977 combinations in RAS mutant/FLT3i-resistant patient-derived xenograft models are ongoing and will be presented. Collectively, our data provide preclinical evidence that combination therapies leveraging RAS MULTI(ON) inhibition are effective in suppressing RAS-mutant AML clones, a common mechanism of resistance to currently approved targeted therapies in AML and a current area of high unmet clinical need.[2] Broad-spectrum RAS inhibition has the potential to benefit roughly a quarter of human patients with cancer whose tumours are driven by RAS mutations. RMC-7977 is a highly selective inhibitor of the active GTP-bound forms of KRAS, HRAS and NRAS, with affinity for both mutant and wild-type variants. More than 90% of cases of human pancreatic ductal adenocarcinoma (PDAC) are driven by activating mutations in KRAS.[3] |
| 分子式 |
C47H60N8O6S
|
|---|---|
| 分子量 |
865.094309806824
|
| 精确质量 |
864.44
|
| 元素分析 |
C, 65.25; H, 6.99; N, 12.95; O, 11.10; S, 3.71
|
| CAS号 |
2765082-12-8
|
| PubChem CID |
164726623
|
| 外观&性状 |
White to light yellow solid powder
|
| LogP |
4.7
|
| tPSA |
172Ų
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
12
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
62
|
| 分子复杂度/Complexity |
1620
|
| 定义原子立体中心数目 |
5
|
| SMILES |
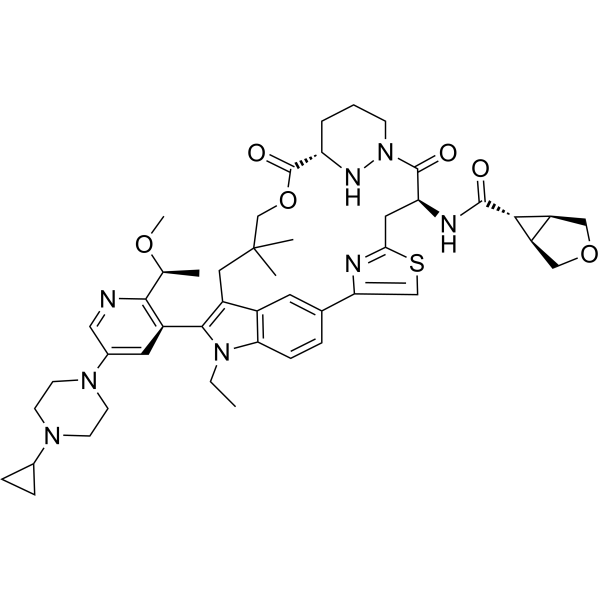
CCN1C2=C3C=C(C=C2)C4=CSC(=N4)C[C@@H](C(=O)N5CCC[C@H](N5)C(=O)OCC(CC3=C1C6=C(N=CC(=C6)N7CCN(CC7)C8CC8)[C@H](C)OC)(C)C)NC(=O)C9[C@H]1[C@@H]9COC1
|
| InChi Key |
NBLZKEHVVJSAAY-OFTZCYAMSA-N
|
| InChi Code |
InChI=1S/C47H60N8O6S/c1-6-54-39-12-9-28-18-31(39)33(43(54)32-19-30(22-48-42(32)27(2)59-5)53-16-14-52(15-17-53)29-10-11-29)21-47(3,4)26-61-46(58)36-8-7-13-55(51-36)45(57)37(20-40-49-38(28)25-62-40)50-44(56)41-34-23-60-24-35(34)41/h9,12,18-19,22,25,27,29,34-37,41,51H,6-8,10-11,13-17,20-21,23-24,26H2,1-5H3,(H,50,56)/t27-,34-,35+,36-,37+,41+/m0/s1
|
| 化学名 |
(1R,5S,6r)-N-((63S,4R,Z)-12-(5-(4-cyclopropylpiperazin-1-yl)-2-((S)-1-methoxyethyl)pyridin-3-yl)-11-ethyl-10,10-dimethyl-5,7-dioxo-61,62,63,64,65,66-hexahydro-11H-8-oxa-2(4,2)-thiazola-1(5,3)-indola-6(1,3)-pyridazinacycloundecaphane-4-yl)-3-oxabicyclo[3.1.0]hexane-6-carboxamide
|
| 别名 |
2765082-12-8; SCHEMBL25774785; RMC-7977; RMC 7977; RMC7977; EX-A7974;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.1559 mL | 5.7797 mL | 11.5595 mL | |
| 5 mM | 0.2312 mL | 1.1559 mL | 2.3119 mL | |
| 10 mM | 0.1156 mL | 0.5780 mL | 1.1559 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。