| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
/Researchers/ found that t-butyl alcohol is eliminated slowly from the blood of rats. t-Butyl alcohol was dissolved in water and a dose of 25 mmol/kg was administered by gastric intubation to female Wistar rats (number unspecified). The t-butyl alcohol blood concentration at 2 hr was 13.24 mM, at 5 hr it was 12.57 mM, and at 20 hr it was 11.35 mM. The purpose of this study was to fully characterize the pharmacokinetics of tertiary butyl alcohol in male and female F-344 rats following intravenous administration of 37.5, 75, 150 and 300 mg/kg TBA. TBA was observed to undergo a rapid distribution phase followed by a slower elimination phase. The steady-state volume of distribution for TBA was roughly 4.5 times greater than total body water, and the clearance was lower than the estimated glomerular filtration rate. The elimination of TBA appears to saturate at higher doses, as evidenced by a disproportional increase in area under the concentration-time curve and decreased rate of clearance. In animals, tert-butanol is absorbed through the lungs and gastrointestinal tract ... . t-Butyl alcohol moves rapidly from the blood into the tissues. Eleven male Sprague-Dawley rats were cannulated and intravenously given 350 mg/kg (14)C-t-butyl alcohol. At numerous times following injection, blood samples were withdrawn and the samples measured for radioactivity. There were two phases in the elimination of (14)C-t-butyl alcohol from the blood. The first was a rapid phase, which probably represented the distribution of (14)C-t-butyl alcohol from the blood to other body tissues. The second represented a first-order elimination of radioactivity from the blood with a half-life of approximately 8 hr, indicating that (14)C-t-butyl alcohol was being eliminated primarily as metabolic product(s). For more Absorption, Distribution and Excretion (Complete) data for T-BUTYL ALCOHOL (9 total), please visit the HSDB record page. Metabolism / Metabolites /Researchers/ administered 12 mmol of t-butyl alcohol by stomach tube to three chinchilla rabbits. t-Butyl alcohol was conjugated to a large extent with glucuronic acid, and glucuronides were readily isolated from the rabbit urine; as a percentage of dose, the average extra glucuronic acid excreted over 24 hr was 24.4%. The researchers suggested that volatile alcohols might also be eliminated to some extent in an unchanged state by the lungs. No aldehydes or ketones were detected in the expired air of a rabbit given 6 mL t-butyl alcohol (route unspecified). t-Butyl alcohol is not a substrate for alcohol dehydrogenase or for the peroxidative activity of catalase, therefore, it is used frequently as an example of a non-metabolizable alcohol. tert-Butyl alcohol is a scavenger of the hydroxyl radical and can be oxidized to formaldehyde and acetone from four different systems; (a) iron catalyzed oxidation of ascorbic acid (b) hydrogen peroxide and iron (c) coupled oxidation of xanthine oxidase, an enzymatic bound system (d) NADPH-dependent microsomal electron transfer, a membrane bound system. Because of its special biochemical properties, t-butyl alcohol may be a valuable probe for the detection of hydroxyl radicals in intact cells and in vivo. In vitro reactions with liver microsomes of mice produced tert-butanol from isobutane. Male Wistar rats exposed to 50, 100, or 300 ppm methyl tertiary-butyl ether vapor ... showed ... blood concns of tert-butanol which were dose dependent indicating metabolic breakdown of the ether in vivo. For more Metabolism/Metabolites (Complete) data for T-BUTYL ALCOHOL (6 total), please visit the HSDB record page. Tert-butanol has known human metabolites that include Tert-butyl hydrogen sulfate and (2S,3S,4S,5R)-3,4,5-Trihydroxy-6-[(2-methylpropan-2-yl)oxy]oxane-2-carboxylic acid. Tert-butanol is a known human metabolite of tert-butyl ethyl ether (ETBE) and tert-butyl methyl ether. Biological Half-Life In Long-Evans rats treated with tert-butanol (1 g/kg body weight, route not specified), the rate of disappearance of tert- butanol from the blood was apparently of first order with a half life of 9.1 hr. Two Sprague-Dawley rats were given 1500 mg/kg (14)C-t-butyl alchol by oral gavage. Their blood was sampled at various times following the dosage. ... There was a half-life of 9 hr similar to that seen following intravenous dosing with 350 mg/kg (14)C-t-butyl alchol. In mice, after a single ip injection of 8.1 mmol tert-butanol/kg body weight, initial blood levels of 8 mmol took 8-9 hr for elimination (blood- tert-butanol half-life was approximately 5 hr). However, after 3 days, inhalation at a vapor concentration to give levels of 8 mmol/L blood, tert-butanol disappeared within 3 hr of removal of mice from the inhalation chamber (half-life of tert- butanol in blood was approximately 1.5 hr). |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Data
LC50 (rat) = 10,000 ppm/4h Interactions Tertiary butyl alcohol and trichloroacetic acid are known to be contaminants in drinking water. In order to evaluate the interactive toxicity of t-butyl alcohol with trichloroacetic acid, young male Wistar rats were dosed through water at a dose level of t-butyl alcohol (TBA)-0.5% (v/v), trichloroacetic acid (TCA)-25 ppm and a combined dose of TBA + TCA (0.5% v/v TBA-25 ppm TCA) for a period of 10 weeks ad libitum and were maintained on normal diet. The control animals received plain water and normal diet. The liver and kidney histology was undertaken to see whether subtoxic administration of TBA and TCA individually as well as combined administration for a period of 10 weeks would bring about any histological alterations. It was observed that TBA, TCA and TBA + TCA caused histological alterations in the liver such as centrilobular necrosis, vacuolation in hepatocytes and loss of hepatic architecture. TBA and TBA + TCA caused periportal proliferation and lymphocytic infiltration. Hypertrophy of hepatocytes in the periportal area was a characteristic feature in the liver of TCA treated rats. Moreover, in the histology of the kidney, in the three treated groups, degeneration of renal tubules, with syncitial arrangements of the nucleus of renal tubular epithelial cells was evident. In addition to this, degeneration of the basement membrane of the Bowmans capsule, diffused glomeruli and vacuolation of glomeruli was also evident in the three treated rat kidneys. Renal tubular proliferation in certain areas was also evident in certain areas of the kidney in TCA treated rats. The results indicate that, TBA and TCA do bring about alterations in histology of liver and kidney, but on combined administration, do not show enhanced toxicity in the form of increased hepatic and renal injury. Non-Human Toxicity Values LD50 Rats oral 3500 mg/kg bw LD50 Rabbit oral 3.6 g/kg bw LD50 Mouse ip 0.9 g/kg bw LD50 Mouse iv 1.5 g/kg bw LD50 Mouse subcutanous 3.9 g/kg bw |
| 参考文献 | |
| 其他信息 |
Tert-butyl alcohol is a colorless oily liquid with a sharp alcohol odor. Floats and mixes with water. Produces irritating vapor. Freezing point is 78 °F. (USCG, 1999)
Tert-butanol is a tertiary alcohol alcohol that is isobutane substituted by a hydroxy group at position 2. It has a role as a human xenobiotic metabolite. It derives from a hydride of an isobutane. tert-Butanol has been reported in Psidium guajava with data available. An isomer of butanol that contains a tertiary butyl group that consists of three methyl groups, each separately attached to a central (tertiary) carbon. See also: tert-Butoxy (annotation moved to). |
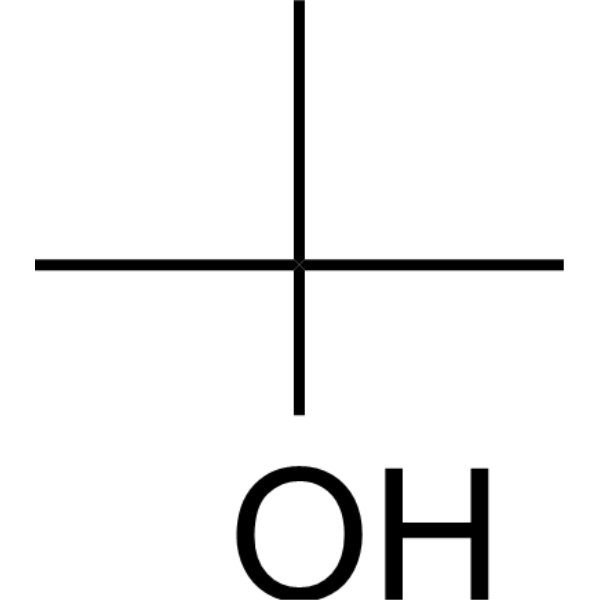
| 分子式 |
C4H10O
|
|---|---|
| 分子量 |
74.12
|
| 精确质量 |
74.073
|
| CAS号 |
75-65-0
|
| PubChem CID |
6386
|
| 外观&性状 |
A colorless liquid, which forms rhombic crystals melting at 25 to 25.5 °C
Colorless liquid or rhombic prisms or plates Crystals Colorless liquid or solid (above 77 °F) [Note: Often used in aqueous solutions]. |
| 密度 |
0.8±0.1 g/cm3
|
| 沸点 |
84.6±8.0 °C at 760 mmHg
|
| 熔点 |
23-26 °C(lit.)
|
| 闪点 |
11.7±0.0 °C
|
| 蒸汽压 |
46.0±0.3 mmHg at 25°C
|
| 折射率 |
1.395
|
| LogP |
0.51
|
| tPSA |
20.23
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
1
|
| 可旋转键数目(RBC) |
0
|
| 重原子数目 |
5
|
| 分子复杂度/Complexity |
25.1
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
DKGAVHZHDRPRBM-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C4H10O/c1-4(2,3)5/h5H,1-3H3
|
| 化学名 |
2-methylpropan-2-ol
|
| 别名 |
Trimethyl carbinol, 99.5%; tert-Butyl alcohol, 99.5%; 2-Methyl-2-propanol, 99.5%
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 13.4916 mL | 67.4582 mL | 134.9164 mL | |
| 5 mM | 2.6983 mL | 13.4916 mL | 26.9833 mL | |
| 10 mM | 1.3492 mL | 6.7458 mL | 13.4916 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。