| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
Glucagon Receptor
|
|---|---|
| 体外研究 (In Vitro) |
Adomeglivant 不能阻断胰岛的 cAMP 升高作用[2]。 Adomeglivant 对 B 族 GPCR 表现出高性,并与 GluR、GLP-1R 和 GIP-R 中的振荡结合基序电位共振[2]。
胰高血糖素和LY2409021都靶向胰高血糖激素和GLP-1受体。 LY2409021阻断GLP-1和Ex-4在GLP-1R上的激动作用。 LY2409021是一种GIP-R拮抗剂,但不能阻断腺苷对A2B受体的作用。 LY2409021阻断GGP817在GluR和GLP-1R上的激动作用。GluR变构抑制剂LY2409021和MK 0893拮抗GLP-1R上的胰高血糖素和GLP-1作用,而des-His1-[Glu9]胰高血糖蛋白拮抗GluR上的血糖素作用,同时对GLP-1R处的胰高葡萄糖素或GLP-1具有最小的抑制作用[2]。 |
| 体内研究 (In Vivo) |
Adomeglivant (5 mg/kg;ip) 在 Avpires-Cre+ 电极中完全消除 CNO (氯氮平-N-氧化物) 的高血压作用。(CNO 是一种心血管的药物兴奋剂,可用于hM3Dq 感应的膜去心肌并增加表达 hM3Dq 的精氨酸加压素 (AVP) 神经元的放电率)[3] 动物模型: Avpires-Cre+ 小鼠[3] 剂量: 5 mg/kg 给药方式: 腹腔注射, CNO 前 30 分钟 结果:完全消除了 CNO 的高血糖作用。
|
| 酶活实验 |
96孔格式的FRET报告分析[2]
将稳定表达重组GPCR的HEK293细胞以80%的融合率铺在涂有大鼠尾胶原的96孔透明底部测定板上。然后在感染多样性相当于每细胞25个病毒颗粒的条件下,以约60000个细胞/孔的密度用H188病毒转导细胞16小时。移除培养基,用200μl/孔的标准细胞外盐水(SES)溶液代替,该溶液补充了11 mm葡萄糖和0.1%BSA。SES的成分(单位为mm)为:138 NaCl、5.6 KCl、2.6 CaCl2、1.2 MgCl2、11.1葡萄糖和10 Hepes(295 mosmol,pH 7.4)。使用配备有激发和发射光单色器的FlexStation 3微孔板阅读器进行FRET的实时动力学分析。激发光在435/9nm(455nm截止)处发出,在485/15nm(青色荧光蛋白)或535/15nm(黄色荧光蛋白)处检测到发射光。发射强度是每个孔每个时间点12次激发闪光的平均值。将溶解在SES中的试验溶液放置在V-bottom 96孔板中,并使用自动移液程序将50μl的每种试验溶液转移到含有这些细胞单层的测定板的每个孔中。计算了每口井的485/535排放比,并对12口井的平均值±标准偏差值进行了平均。使用基线减法对这些FRET比值进行归一化,使得y轴值0对应于初始基线FRET比值,而值100对应于FRET比值的100%增加(即加倍)。将数据导出到Origin 8.0后,绘制了ΔFRET比率的时间过程。Origin 8.0也用于非线性回归分析,以量化剂量反应关系。 |
| 细胞实验 |
使用以150000个受体/细胞的密度稳定表达人GLP-1R的HEK293细胞。从T.P.Sakmar、a.M.Cypess和C.G.Unson获得以250000受体/细胞的密度稳定表达大鼠GlucR的HEK293细胞。从T.J.Kieffer获得以尚未确定的受体密度稳定表达大鼠GIP-R的HEK293细胞。稳定表达H188的HEK293细胞由O.G.Chepurny在Holz实验室产生。所有细胞培养物均保存在含有25mm葡萄糖的Dulbecco改良Eagle培养基中,并补充了10%胎牛血清和1%青霉素/链霉素。在37°C的加湿培养箱中平衡的细胞培养物,用5%的二氧化碳充气,每周传代一次[2]。
|
| 动物实验 |
AAV-DIO-hM3Dq-mCherry was injected bilaterally into the supraoptic nucleus (SON) of Avpires-Cre+ mice. Mice were fasted for 4 hours (beginning at 10:00 am), and then CNO (or saline vehicle) was injected (3 mg/kg i.p.). In the same cohort, during a different trial, the glucagon receptor antagonist Adomeglivant (LY2409021)Adomeglivant (LY2409021)
AAV-DIO-hM3Dq was injected into ThCre+ mice, targeting A1/C1 neurons. CNO (1 mg/kg) was then injected (i.p.). Antagonists (or vehicle) for the V1bR (SSR149415, 30 mg/kg) or glucagon receptor (GCGR; Adomeglivant (LY2409021)
|
| 药代性质 (ADME/PK) |
Prediction of pharmacokinetics of CAA and PIB in human body and comparison of the results with the already known antagonists [1]
Certain structural and molecular features of compounds govern their pharmacokinetic properties in our body. Qikprop module of Schrodinger was used to evaluated the drug likeliness of all the four inhibitors. The obtained values for molecular weight, number of hydrogen bond donors, number of hydrogen bond acceptors and logP were used to assess violation of Lipinski's rule of five if any. To further account for the potential of the compounds to act as efficient drug candidates, their absorption, distribution, metabolism and excretion (ADME) properties were also calculated in silico using Qikprop. Physico-chemical properties and pharmacokinetics of GCGR inhibitors- CAA, PIB, MK-0893, LY2409021 [1] To supplement the information gained from binding affinity prediction, Qikprop was used to calculate various other physically significant descriptors and pharmaceutically relevant properties of these small molecules. Qikprop predicts these molecular properties and provides significant ranges for comparing their values with those of 95% of already known pharmaceutical drugs. The descriptor, "#star" denotes the number of outlying properties of the molecule i.e., the properties which do not fall within the range of values for already known drugs. So, lesser the number better is the druglikeness of the small molecule. MK-0893, which showed the highest binding affinity, had a #star value of 4 whereas all the other three compounds had 0 #star. Hence, except for MK-0893, the computed properties for the other three compounds did not lie outside the required range and were very similar to that of the known drugs. Lipinski's rule of five is a thumb rule which determines the likeliness of a drug to be orally active based on four molecular properties. Table 1 lists the values of all four properties for these four compounds. MK-0893 with a molecular weight of 588.48 and logP value of 8.18 was not satisfying the lipinski's rule (molecular weight < 500, no. of hydrogen bond donors < 5, no. of hydrogen bond acceptors < 10, logP < 5). Solvent accessible surface area (SASA) and especially polar surface area (PSA) dictate the passive transport of molecules through membranes thereby giving an estimate about the transport properties of the drugs. The total SASA for MK-0893, CAA, PIB and LY2409021 was well within the range given by QikProp. Using some knowledge based set of rules Qikprop also calculates the percentage probability of the drug getting orally absorbed in the human body. This value has been shown to correlate well with the human oral absorption. PIB showed the highest oral absorption with a percentage value of 100%. Out of rest three, LY2409021 had the least value of 28.78 %. Central nervous system activity is another parameter that needs to be considered for assessing the safety issue. CAA was found to be highly CNS inactive whereas PIB was predicted to possess some minimal amount of CNS activity. Blood brain barrier (BBB) separates the human brain from the direct contact of circulatory system, thus protecting the brain for unwanted solute particles. Both the predicted compounds were shown to be BBB negative ensuring their administration safe for the brain. Even though MK-0893 had higher affinity for GCGR, CAA and PIB were found to be better than this known inhibitor in many aspects. The new compounds showed better druglikeness with acceptable values of ADME properties. This clearly delineates the distinctive potential of CAA and PIB as prospective lead inhibitors of GCGR for the treatment of type II diabetes mellitus. |
| 参考文献 |
|
| 其他信息 |
Objective: Type 2 diabetes (T2D) pathophysiology includes fasting and postprandial hyperglucagonemia, which has been linked to hyperglycemia via increased endogenous glucose production (EGP). We used a glucagon receptor antagonist (LY2409021) and stable isotope tracer infusions to investigate the consequences of hyperglucagonemia in T2D.
Design: A double-blinded, randomized, placebo-controlled crossover study was conducted.
Methods: Ten patients with T2D and ten matched non-diabetic controls underwent two liquid mixed meal tests preceded by single-dose administration of LY2409021 (100 mg) or placebo. Double-tracer technique was used to quantify EGP. Antagonist selectivity toward related incretin receptors was determined in vitro.
Results: Compared to placebo, LY2409021 lowered the fasting plasma glucose (FPG) from 9.1 to 7.1 mmol/L in patients and from 5.6 to 5.0 mmol/L in controls (both P < 0.001) by mechanisms involving reduction of EGP. Postprandial plasma glucose excursions (baseline-subtracted area under the curve) were unaffected by LY2409021 in patients and increased in controls compared to placebo. Glucagon concentrations more than doubled during glucagon receptor antagonism. The antagonist interfered with both glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide receptors, complicating the interpretation of the postprandial data.
Conclusions: LY2409021 lowered FPG concentrations but did not improve postprandial glucose tolerance after a meal in patients with T2D and controls. The metabolic consequences of postprandial hyperglucagonemia are difficult to evaluate using LY2409021 because of its antagonizing effects on the incretin receptors.[4]
Adomeglivant has been investigated for the basic science of Type 2 Diabetes. ADOMEGLIVANT is a small molecule drug with a maximum clinical trial phase of II (across all indications) and has 3 investigational indications. Background: Interaction of the small peptide hormone glucagon with glucagon receptor (GCGR) stimulates the release of glucose from the hepatic cells during fasting; hence GCGR performs a significant function in glucose homeostasis. Inhibiting the interaction between glucagon and its receptor has been reported to control hepatic glucose overproduction and thus GCGR has evolved as an attractive therapeutic target for the treatment of type II diabetes mellitus. Results: In the present study, a large library of natural compounds was screened against 7 transmembrane domain of GCGR to identify novel therapeutic molecules that can inhibit the binding of glucagon with GCGR. Molecular dynamics simulations were performed to study the dynamic behaviour of the docked complexes and the molecular interactions between the screened compounds and the ligand binding residues of GCGR were analysed in detail. The top scoring compounds were also compared with already documented GCGR inhibitors- MK-0893 and LY2409021 for their binding affinity and other ADME properties. Finally, we have reported two natural drug like compounds PIB and CAA which showed good binding affinity for GCGR and are potent inhibitor of its functional activity. Conclusion: This study contributes evidence for application of these compounds as prospective small ligand molecules against type II diabetes. Novel natural drug like inhibitors against the 7 transmembrane domain of GCGR have been identified which showed high binding affinity and potent inhibition of GCGR. [1] G protein-coupled receptors (GPCRs) for glucagon (GluR) and glucagon-like peptide-1 (GLP-1R) are normally considered to be highly selective for glucagon and GLP-1, respectively. However, glucagon secreted from pancreatic α-cells may accumulate at high concentrations to exert promiscuous effects at the β-cell GLP-1R, as may occur in the volume-restricted microenvironment of the islets of Langerhans. Furthermore, systemic administration of GluR or GLP-1R agonists and antagonists at high doses may lead to off-target effects at other receptors. Here, we used molecular modeling to evaluate data derived from FRET assays that detect cAMP as a read-out for GluR and GLP-1R activation. This analysis established that glucagon is a nonconventional GLP-1R agonist, an effect inhibited by the GLP-1R orthosteric antagonist exendin(9-39) (Ex(9-39)). The GluR allosteric inhibitors LY2409021 and MK 0893 antagonized glucagon and GLP-1 action at the GLP-1R, whereas des-His1-[Glu9]glucagon antagonized glucagon action at the GluR, while having minimal inhibitory action versus glucagon or GLP-1 at the GLP-1R. When testing Ex(9-39) in combination with des-His1-[Glu9]glucagon in INS-1 832/13 cells, we validated a dual agonist action of glucagon at the GluR and GLP-1R. Hybrid peptide GGP817 containing glucagon fused to a fragment of peptide YY (PYY) acted as a triagonist at the GluR, GLP-1R, and neuropeptide Y2 receptor (NPY2R). Collectively, these findings provide a new triagonist strategy with which to target the GluR, GLP-1R, and NPY2R. They also provide an impetus to reevaluate prior studies in which GluR and GLP-1R agonists and antagonists were assumed not to exert promiscuous actions at other GPCRs. [2] Hypoglycaemia is a major barrier to the treatment of diabetes. Accordingly, it is important that we understand the mechanisms regulating the circulating levels of glucagon – the body’s principle blood glucose-elevating hormone which is secreted from alpha-cells of the pancreatic islets. In isolated islets, varying glucose over the range of concentrations that occur physiologically between the fed and fuel-deprived states (from 8 to 4 mM) has no significant effect on glucagon secretion and yet associates with dramatic changes in plasma glucagon in vivo. The identity of the systemic factor that stimulates glucagon secretion in vivo remains unknown. Here, we show that arginine-vasopressin (AVP), secreted from the posterior pituitary, stimulates glucagon secretion. Glucagon-secreting alpha-cells express high levels of the vasopressin 1b receptor (V1bR). Activation of AVP neurons in vivo increased circulating AVP, stimulated glucagon release and evoked hyperglycaemia; effects blocked by pharmacological antagonism of either the glucagon receptor or vasopressin 1b receptor. AVP also mediates the stimulatory effects of dehydration and hypoglycaemia produced by exogenous insulin and 2-deoxy-D-glucose on glucagon secretion. We show that the A1/C1 neurons of the medulla oblongata, which are known to be activated by hypoglycaemia, drive AVP neuron activation in response to insulin-induced hypoglycaemia. Hypoglycaemia also increases circulating levels of copeptin (derived from the same pre-pro hormone as AVP) in humans and this hormone stimulates glucagon secretion from isolated human islets. In patients with type 1 diabetes, hypoglycaemia failed to increase both plasma copeptin and glucagon. These findings provide a new mechanism for the central regulation of glucagon secretion in both health and disease.[3] |
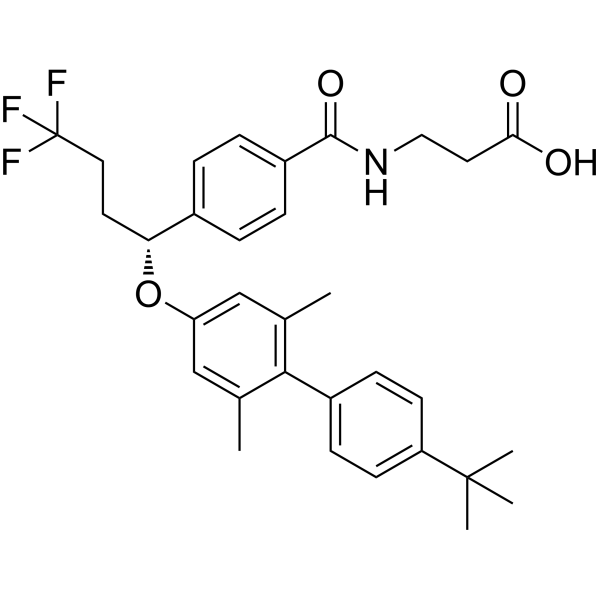
| 分子式 |
C32H36F3NO4
|
|---|---|
| 分子量 |
555.63
|
| 精确质量 |
555.259
|
| 元素分析 |
C, 69.17; H, 6.53; F, 10.26; N, 2.52; O, 11.52
|
| CAS号 |
872260-19-0
|
| 相关CAS号 |
872260-47-4 (racemic); 1488363-78-5; 488363-78-5 (S-isomer); 872260-19-0 (R-isomer)
|
| PubChem CID |
91936837
|
| 外观&性状 |
Typically exists as solids at room temperature
|
| LogP |
7.53
|
| tPSA |
75.6
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
11
|
| 重原子数目 |
40
|
| 分子复杂度/Complexity |
798
|
| 定义原子立体中心数目 |
1
|
| InChi Key |
FASLTMSUPQDLIB-HHHXNRCGSA-N
|
| InChi Code |
InChI=1S/C32H36F3NO4/c1-20-18-26(19-21(2)29(20)23-10-12-25(13-11-23)31(3,4)5)40-27(14-16-32(33,34)35)22-6-8-24(9-7-22)30(39)36-17-15-28(37)38/h6-13,18-19,27H,14-17H2,1-5H3,(H,36,39)(H,37,38)/t27-/m1/s1
|
| 化学名 |
3-[[4-[(1R)-1-[4-(4-tert-butylphenyl)-3,5-dimethylphenoxy]-4,4,4-trifluorobutyl]benzoyl]amino]propanoic acid
|
| 别名 |
(+)-LY2409021; Adomeglivant, (+)-; RIM88PH2RA; UNII-RIM88PH2RA; 872260-19-0; (+)-adomeglivant;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7998 mL | 8.9988 mL | 17.9976 mL | |
| 5 mM | 0.3600 mL | 1.7998 mL | 3.5995 mL | |
| 10 mM | 0.1800 mL | 0.8999 mL | 1.7998 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。