| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
DCAF1; BRD4; drug-linker conjugate
|
|---|---|
| 体外研究 (In Vitro) |
异双官能化合物和分子胶诱导的靶向蛋白质降解为化学探针和药物发现提供了一条令人兴奋的途径。迄今为止,只发现了有限数量的E3连接酶的小分子配体,这是实现靶向蛋白质降解全部潜力的重要限制因素。我们在这里报告了通过化学蛋白质组学发现氮杂环丁烷丙烯酰胺与E3连接酶底物受体DCAF1中的半胱氨酸(C1113)立体选择性和位点特异性反应。我们证明,DCAF1的氮杂环丁烷丙烯酰胺配体可以开发成亲电蛋白水解靶向嵌合体(PROTAC),介导人类细胞中靶向蛋白的降解。我们表明,这一过程是立体选择性的,在表达DCAF1 C1113A突变体的细胞中不会发生。机制研究表明,仅需要DCAF1的低分数参与来支持亲电PROTAC的蛋白质降解。这些发现共同证明了立体化学定义的亲电化合物集的化学蛋白质组学分析如何揭示E3连接酶上支持靶向蛋白质降解的可连接位点[1]。
|
| 参考文献 | |
| 其他信息 |
Here, we have described the chemical proteomic discovery of a ligandable cysteine (C1113) in the E3 ligase substrate receptor protein DCAF1 and the conversion of azetidine acrylamides targeting this cysteine into electrophilic PROTACs. These findings are significant because, to our knowledge, they describe the first electrophilic PROTACs that have been shown to act in a stereo- and site-selective manner. The properties of stereoselectivity and site-specificity provide convenient ways to establish controls to verify on-target activity for electrophilic PROTACs, as we have shown herein for DCAF1 and others have demonstrated for reversibly binding PROTACs that engage VHL in a stereoselective manner. We further interpret these features to indicate the presence of a high-quality druggable pocket in proximity to DCAF1_C1113. Also supportive of this conclusion is the recent report of a noncovalent DCAF1 ligand that binds a pocket near C1113 (PDB code: 7SSE, Figure S10). While we have taken advantage of the druggability of DCAF1_C1113 to create electrophilic PROTACs, we also imagine that more advanced covalent ligands might serve as molecular glues or antagonists of the various physiological and pathological functions of DCAF1. Indeed, small-molecule antagonists of DCAF1 could counteract the pathogenesis of viruses like HIV-1 and HIV-2 that co-opt this E3 ligase substrate receptor to suppress immune cell responses. More generally, the rich dataset of stereoselective interactions reported herein between azetidine acrylamides and cysteines in the human T-cell proteome (Figure 1B and Dataset S1) should offer attractive starting points for chemical probe discovery for additional proteins from structurally and functionally diverse classes.[1]
As has been described previously, electrophilic PROTACs have the potential to maximally leverage the catalytic potential of targeted protein degradation by creating “neo”-E3 ligases that are permanently modified (until physical turnover) with a substrate-binding compound. However, key variables can impact the success of such endeavors, including the half-life and substrate compatibility of the E3 ligase, as well as the quality of the chemical probes that target it. On the latter point, we view the azetidine acrylamide reactive group found herein to stereoselectively engage DCAF1_C1113 as an encouraging starting point for probe optimization, especially in comparison to electrophilic PROTACs reported to target other E3 ligases, which mostly use high-reactivity groups such as α-chloroacetamides. Nonetheless, we have, so far, only observed targeted protein degradation for electrophilic PROTACs in cells expressing recombinant DCAF1, and it will be important in future studies to determine if this activity can be extended to endogenous DCAF1. That we observed evidence of electrophilic PROTACs promoting a ternary complex with endogenous DCAF1 and FBKP12, as well as inducing FKBP12 ubiquitination, is encouraging, even though these effects were not apparently robust enough to lead to substantial FKBP12 degradation in cells expressing endogenous DCAF1 (possibly due to counteracting cellular deubiquitinases). Further improvements in electrophilic PROTAC performance may require greater levels of cellular engagement of DCAF1, as our first-generation compounds only appear to modify ≤ 20% of recombinant DCAF1 at functional concentrations in cells. A recent study has also described an autoinhibitory oligomerization mechanism for DCAF1 that may not be shared by other CRL4 substrate receptors58. If a substantial proportion of endogenous DCAF1 is in an autoinhibited tetrameric state, then a greater quantity of total DCAF1 may need to be modified by electrophilic PROTACs to support targeted protein degradation. On the other hand, we wonder whether this autoregulatory feature of DCAF1 might also be exploited to create electrophilic PROTACs and/or molecular glues that carry out context-dependent protein degradation only in those cell types where DCAF1 is in an activated state.[1] |
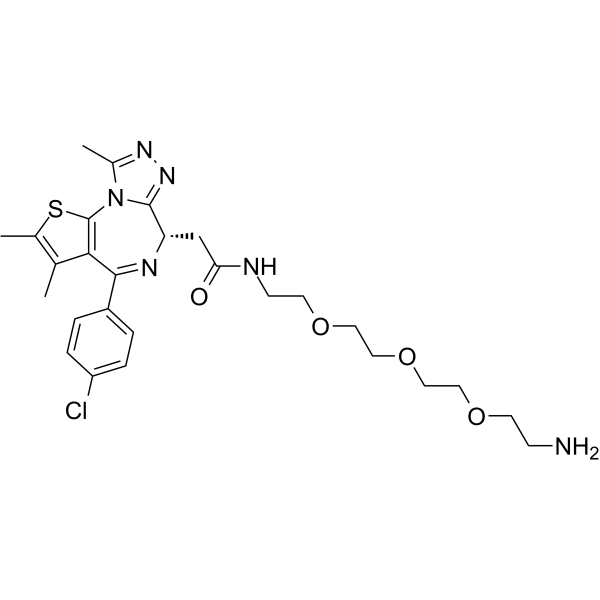
| 分子式 |
C27H35CLN6O4S
|
|---|---|
| 分子量 |
575.12
|
| CAS号 |
2098790-24-8
|
| 相关CAS号 |
2241669-80-5
|
| 外观&性状 |
Typically exists as solids at room temperature
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7388 mL | 8.6938 mL | 17.3877 mL | |
| 5 mM | 0.3478 mL | 1.7388 mL | 3.4775 mL | |
| 10 mM | 0.1739 mL | 0.8694 mL | 1.7388 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。