| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg | |||
| 500mg | |||
| Other Sizes |
| 靶点 |
mGluR
|
|---|---|
| 体外研究 (In Vitro) |
MCPG 可以燃烧 I 组 (mGluR1 和 mGluR5) 和 II 组受体 (mGluR2 和 mGluR3)[4]。(S)-MCPG (100 μM) 对 WT 切片培养中的脊柱产生或去除机制没有明显影响。 (S)-MCPG 抑制 TBS 诱导的脊柱更新增加,并干扰海马切片培养物中活动依赖性中枢稳定机制 [1]。
|
| 体内研究 (In Vivo) |
(RS)- MCPG (α- MCPG;500 μM) 沸腾幼年或新生神经元中 TBS 感应的 EGABA 变化[3]。 用低剂量 (RS)- MCPG (25 nM;ic;每天;5 天) 预处理可显着着解除10日龄卡车和雌性Sprague-Dawley血统支架中苯丙胺感应的运动活动[4]。
代谢型谷氨酸受体拮抗剂部分逆转Fmr1 KO小鼠的脊柱动力学缺陷[1] 然后,我们研究了这些缺陷是否可以逆转,并测试了代谢型谷氨酸受体拮抗剂MCPG(100μm)对脊柱动力学的影响。在WT切片培养中,MCPG对基底棘的形成或消除机制没有可检测到的影响(图3A和B)。然而,它阻止了TBS引发的脊柱翻转增加(图3A和B;新脊柱:23.8±3.3%,n=6233对42.4±7.9%,n=5240,P<0.05;失去的脊柱:20.8±2.4%对43.7±8.8%,P<0.05)。它还干扰了活动依赖性脊柱稳定的机制。在野生型小鼠中,MCPG显著降低了TBS后增大与非增大脊柱的差异稳定性(图3C;圆圈:ns:无显著差异,双向方差分析),这与代谢型受体参与LTP诱导机制的证据一致(Anwyl,2009)。在Fmr1 KO小鼠中,MCPG有三个主要作用。首先,它通过每24小时增强新形成和丢失的棘突来恢复基础代谢水平(图3A和B:新棘突:17.0±3.2%对9.3±1.3%,n=6,8,P<0.05;丢失棘突:15.2±2.5%对9.5±1.5%,n=6,8%,P<0.05),这与Fmr1 KO小鼠中观察到的代谢减少可能是由于代谢受体过度激活的观点一致(Pfeiffer&Huber,2007)。其次,它降低了脊柱翻转对活动的敏感性增加:TBS后新脊柱的形成仍然增加,但程度较小(图3A;27.2±3.6%,n=5个细胞,237个脊柱对15.6±3.0%,n=7274个脊柱,P<0.05)。然而,TBS刺激后脊柱损失的变化没有达到统计学意义(图3B;P=0.20)。因此,MCPG使基础脊柱动力学正常化,并降低了脊柱翻转对活动的敏感性。第三,MCPG提高了脊柱的总体稳定性[图3D;与(圆形)和没有MCPG(方形)相比,P<0.05,双向方差分析]。然而,MCPG未能改善活动依赖性脊柱稳定的缺陷。TBS后,扩大和非扩大的脊柱表现出相同的稳定性,扩大的脊柱没有优先的稳定性(图3D;圆圈,ns:不显著,n=5个细胞,分析了237个脊柱)。因此,MCPG改善了脊柱的总体稳定性,但没有恢复活动依赖性脊柱稳定的机制。[1] 在先前植入套管的大鼠中,研究了代谢型谷氨酸受体拮抗剂(+)-α-甲基-4-羧基苯甘氨酸(MCPG)对水迷宫和特定情境联想学习表现的影响。在测试前经脑室注射(i.c.v.)MCPG(20.8微克)会损害大鼠在Morris水迷宫空间版本中的表现,但该剂量的1/10不会。训练后24小时评估的记忆保持能力也受到高剂量MCPG的影响。然而,除了药物对感知能力的影响外,高剂量并没有损害提示版水迷宫的表现。MCPG对另一项海马依赖性空间学习任务(情境依赖性恐惧条件反射任务)的表现的影响得到了进一步的表征。MCPG(20.8微克,静脉注射)不会干扰本任务中的条件性冷冻。为了进行比较,一组大鼠注射了NMDA受体阻断剂MK801。与对照组相比,MK801在扰乱Morris水迷宫空间版本性能的剂量(0.08 mg/kg)下显著降低了冷冻。这些实验表明,MCPG敏感的代谢型受体可能只需要用于有限的空间学习任务子集,而NMDA受体可能在所有空间学习中发挥不可或缺的作用。[2] 莫里斯水迷宫[2] 在4天的训练中,接受MCPG治疗(高剂量)和对照组动物的表现都有显著改善,表现为到达平台的时间[F(3108)=107.5;p<0.01]和动物与平台的距离[F(310b)=77.4;p<0.01]都有所减少(图1)。然而,给予MCPG的动物学习这项任务的速度明显较慢。MCPG治疗的大鼠到达平台的时间更长[F(1,35)=9.6;p<0.01],在训练期间,它们与平台的距离也比载体注射的动物长[F(1,3,5)=9.9;p<0.01)。在第2天和第3天,MCPG大鼠的逃避潜伏期明显更长(p<0.01,图1(a)),在前3天的每一天,它们的搜索错误测量值都明显更长(p<0.01;图1(B),Newman-Keuls事后测试)。在第4天,MCPG治疗的动物达到了与对照组动物相同的性能水平。 情境恐惧制约[2] 作为学习的另一种衡量标准,我们在特定情境的联想学习中测试了MCPG对大鼠组的影响。在恐惧条件反射的训练阶段,将大鼠置于电击室中,在为期2天的6分钟训练中进行三次脚踢。24小时后,将大鼠送回电击室,并监测其冷冻行为。红外光电池监测动物的活动,在测试阶段,摄像机记录了实验过程,供实验人员离线审查。 图5(A)显示了训练第一天动物的平均活动。由于休克的发生,两组的活动都降低了[F(5,70)=5.6;p<0.01],但两组之间没有统计学差异。与对照组相比,在第3天的测试中,运动活动和冷冻都不受MCPG的影响(图5(B))。两组在第一分钟后冻结的频率更高,在6分钟疗程结束时冻结的频率更低[F(5,70)=7.6;p<0.01]。这些结果表明,MCPG 20.8μg在空间学习水迷宫中造成的损伤与对情境学习的类似影响无关。 |
| 细胞实验 |
共聚焦成像和电生理学[1]
转染四天后,使用奥林巴斯Fluoview共聚焦显微镜对切片培养物进行重复成像,如所述(Dubos等人,2012)。在0、5、24、48和72小时时,观察辐射层CA1锥体神经元顶端树突上的第二或第三树突段(35-45μm)。使用Osirix软件分析Z-stack图像,并与另一名实验者进行交叉检查。所有突出物都包括在分析中,稳定性被评估为以后仍然存在的刺的比例。如果脊柱头部宽度在0到5小时的时间点之间增加了0.1μm以上,则认为脊柱扩大。所有数据均以n为分析的树突节段数量给出,考虑每个神经元一个树突节段和每个切片培养物一个神经元。在适当的时候,我们还给出了实验池中分析的刺的总数。 对于电生理记录,将海马培养物或急性切片(4-6周龄的小鼠)保存在所述的界面室中(De Roo等人,2008b),灌注在具有以下成分(单位为mm)的培养基中:NaCl,124;氯化钾1.6;氯化镁,1.5;氯化钙,2.5;KH2PO4,1.2;碳酸氢钠24;葡萄糖,10;抗坏血酸,2;用95%的O2和5%的CO2鼓泡。Theta爆发刺激(TBS)由5个5 Hz的序列组成,每个序列由4个100 Hz的脉冲组成,以10秒的间隔重复三次。使用IGOR软件测量场兴奋性突触后电位斜率和振幅。 蛋白质印迹[1] 在体外维持12-14天并用15nm BpV处理的海马切片培养物中,以及在腹腔注射BpV[30μg/100g生理盐水(200μL)]的4-6周龄小鼠上,测试了BpV对S473位点AKT磷酸化的影响。分别在切片和动物处理后30分钟或1-4小时,使用含有(以毫米计):NaCl,150的溶液在冰上裂解组织;Tris-HCl,50,pH 7.4;EDTA,2;二硫苏糖醇,1;1%Triton X-100,10%甘油,完整蛋白酶抑制剂片剂,含磷酸酶抑制剂(单位:mm):原钒酸钠,1;对硝基苯磷酸酯,20;β-甘油磷酸酯,20;冈田酸,50 ng/mL。将裂解物超声处理,并将30μg总蛋白放在Nupage 4–12%Bis-Tris凝胶上。凝胶在3-(N-吗啉代)丙磺酸十二烷基硫酸钠缓冲液中运行,并转移到硝化纤维膜上。使用1:3000稀释的兔抗磷酸Ser473单克隆抗体进行磷酸化AKT免疫印迹。通过β-actin免疫印迹对60kDa的pAKT带进行密度分析,并将其表示为WT值的函数(单克隆抗体,1:20000)。 |
| 动物实验 |
Animal/Disease Models: Morris water maze learning in male Lister-Hooded rats [2]
Doses: 20 μg Route of Administration: intracerebroventricular injection; 20 μg; 4 days Experimental Results: Impaired rat performance in the spatial version of the Morris water maze , but 1/10 of that dose did not. Animals and Morris water maze [1] Twenty male adult Fmr1-KO mice (FVB.129P2-Pde6b+ Tyrc-ch Fmr1tm1Cgr/J) and 20 male adult WT controls (FVB.129P2-Pde6b+ Tyrc-ch/AntJ) were used. Mice were individually identified and housed according to the Swiss legislation on animal protection. One Fmr1-KO and one WT mice were used for pilot tests; the final sample (19 Fmr1-KO and 19 WT) was divided into two groups: nine mice were treated with BpV and 10 received the vehicle. The mice were trained in a Morris water maze with four daily trials during four consecutive days. At day 5, a probe trial was run in the absence of the escape platform. Immediately after, mice received either an i.p. injection of BpV [30 μg/100 g in saline (200 μL)] or an i.p. injection of 200 μL saline. One hour later, all mice started a novel, four-trial training session with the platform in the opposite quadrant (reversal learning). Reversal training continued for two more days; treatment with BpV or saline was repeated accordingly. At day 8, a second probe test was run. Swim paths were videorecorded and analysed by a videotracking system. Variables assessed were escape latencies, times spent in target quadrants and platform place proximity indices (PIs), calculated by measuring the average distances to the platform [(PI probe1 − PI probe2)/(PI probe1 + PI probe2)×100]. Animals were allowed at least 7 days to recover from surgery and were handled daily during this period. They were housed individually for the remainder of the experiment. At the time of behavioral testing, the xstylets were replaced by 33-gauge injection needles connected to Hamilton microsyringes. MCPG or vehicle was administered intraventricularly (i.c.v.) in a volume of 5 μl delivered at a rate of approximately 1 μl/min. The needle was left in place for an additional 1–2 min after completion of the injection to allow diffusion of the drug. The high dose (20.8 μg) or the low dose (2.1 μg) of MCPG were prepared daily and stored at room temperature. At the end of each experiment cannula placements were examined by Nissl staining. [2] Behavioral methods [2] Morris water maze/spatial task [2] Two experiments using the metabotropic glutamate antagonist MCPG were conducted with this procedure. In the first experiment, the high dose of MCPG (20.8 μg) was used. Thirty-six rats were trained in the Morris water maze (Morris, 1984), consisting of a circular pool, 120 cm diameter, filled with water rendered opaque by milk and maintained at 26±1°C. The wall of the pool, 60 cm higher than the water level, was painted white. A platform, 10 cm in diameter and submerged by 2–3 cm, represented the escape from the water. The day before testing, animals were given 2 min each of free swimming in the pool to acclimatize them to the water. The rats were placed in a different starting position (among six possible) in each trial. The platform was always left in a fixed location throughout the 4-day training experiment. Animals were allowed 90 sec to locate the platform. Rats that had not found the platform after 90 sec were placed on it and allowed to remain there for the 60 sec intertrial time. Animals were given 4 trials a day for 4 consecutive days. MCPG (n=18) or vehicle (n=18) was injected i.c.v. 5 min before the start of the session every day. A computer-assisted program measured the path and time taken by each animal to reach the platform. While swimming, the proximity of the animal's position with respect to the target was also analyzed by the computer system. This measure, called the “search error”, represents the corrected cumulative distance from the escape platform (Gallagher et al., 1993). A proximity measure was calculated each second by averaging the distance between the rat and the target platform 10 times per second. A correction procedure was used to account for the six different starting points. Cumulative distance was employed on training trials and mean distance from the target was used on the fifth day during the probe trial (for more detail, see Gallagher et al., 1993). Twenty-four hours after the last training session, the animals were tested in a single probe trial for retention of spatial memory. Rats were divided into four groups before the probe trial: one group (MCPG/MCPG) was injected with a further dose of MCPG as on previous days (n=9); a second group (n=9) (MCPG/vehicle) had been injected with MCPG during the previous training sessions but received vehicle injection before the probe trial; a third group (vehicle/vehicle) received vehicle as it did in the past days (n=9); and the final group (vehicle/MCPG) was injected with MCPG after receiving only vehicle before (n=9), to test possible acute effects of MCPG on memory retention. The platform was removed from the pool in this trial, and the rats were allowed to swim for 20 sec. Time spent in each quadrant of the pool (Morris et al., 1986) and the averaged distance of the animal from where the target had been previously (Gallagher et al., 1993), were used to estimate memory retention. A second experiment was run with a new group of rats previously implanted with cannulae into the right lateral ventricle. A dose of MCPG (2.1 μg) 10 times lower than in the previous experiment was used, but all the procedures were identical. MCPG (n=8) or vehicle (n=10) was injected i.c.v. 5 min before each daily session consisting of 4 trials. On day 5 the platform was removed and the animals were tested for memory retention. The MCPG group had only eight animals, because two rats in this group became ill during the course of the experiment and were excluded. Morris water maze/cued taskk [2] Three weeks after the end of the spatial task experiment, some of the rats were re-tested in a cued version of the water maze. A 30 cm ×5 cm black tape was placed along the wall of the tank 15 cm above water level and centered around the submerged platform (see inset of Fig. 4). The tape represented the visual cue directing the animals to locate the platform. The tape and platform were left in the same location during each session, but they were moved to a new position every day. Their spatial relationship, however, was always maintained. The animals received 4 trials daily for 4 consecutive days. On the fifth day a probe trial was run as in the spatial task experiment. MCPG (20.8 μg, n=7) or vehicle (n=6) rats were injected i.c.v. every day 5 min before the session. The animals tested in this experiment had been used previously in the spatial version of the water maze. However, most of the animals that we employed had been treated with vehicle in the previous experiment. Contextual fear conditioningk [2] Groups of rats were placed individually in a rodent conditioning chamber (25×20×17 cm, San Diego Instr.) with a ventilation fan providing background noise. Inside the chamber eight infrared beams, 3 cm apart and 0.5 cm above the grid floor, monitored the animal activity. Shock delivery was controlled by a computer. A video-camera placed in front of the chamber provided recordings of every session for off-line behavioral analyses. The shock was a brief (1 sec, 0.5 mA) delivery of direct current produced by a grid floor shocker. On days 1 and 2, conditioning sessions consisted of three presentations of the shock during a 6-min session. On the third day, animals were placed again in the chamber but no shock was delivered. Motor activity was monitored by the computer system, which measured the number of beams interrupted by the animals in each of 12 30 sec intervals. Freezing, used as an index of conditioned fear, was assessed by an experimenter analyzing the videotapes after the experiment. Freezing was defined as the absence of all movement except respiratory-related movements (Phillips and LeDoux, 1992). The per cent of time spent freezing was calculated for each 1-min interval. Two different experiments were conducted. In the first experiment, rats received either vehicle (n=7) or MCPG (20.8 μg, n=7) i.c.v. 5 min before the start of the session for the 3-day experiment. In the second experiment, different groups of rats were injected s.c. with MK801 (n=8) or vehicle (n=8) 30 min before testing. Drugsk [2] (+)-α-Methyl-4-carboxyphenylglycine (MCPG) was employed in all experiments. MCPG (2.08 mg) was dissolved in equimolar NaOH (1 M), diluted to final volume with saline (0.9% NaCl) and the pH was then adjusted to 7.6±0.2. MCPG or vehicle was injected i.c.v. in 5 μl volume to each implanted animal. |
| 参考文献 |
|
| 其他信息 |
(S)-alpha-methyl-4-carboxyphenylglycine is a non-proteinogenic alpha-amino acid that is alanine in which the alpha-hydrogen is replaced by a 4-carboxyphenyl group (the S-enantiomer). It is a non-selective group I/group II metabotropic glutamate receptor (mGluR) antagonist. It has a role as a metabotropic glutamate receptor antagonist.
Fragile X syndrome (FXS) is characterized by intellectual disability and autistic traits, and results from the silencing of the FMR1 gene coding for a protein implicated in the regulation of protein synthesis at synapses. The lack of functional Fragile X mental retardation protein has been proposed to result in an excessive signaling of synaptic metabotropic glutamate receptors, leading to alterations of synapse maturation and plasticity. It remains, however, unclear how mechanisms of activity-dependent spine dynamics are affected in Fmr knockout (Fmr1-KO) mice and whether they can be reversed. Here we used a repetitive imaging approach in hippocampal slice cultures to investigate properties of structural plasticity and their modulation by signaling pathways. We found that basal spine turnover was significantly reduced in Fmr1-KO mice, but markedly enhanced by activity. Additionally, activity-mediated spine stabilization was lost in Fmr1-KO mice. Application of the metabotropic glutamate receptor antagonist α-Methyl-4-carboxyphenylglycine (MCPG) enhanced basal turnover, improved spine stability, but failed to reinstate activity-mediated spine stabilization. In contrast, enhancing phosphoinositide-3 kinase (PI3K) signaling, a pathway implicated in various aspects of synaptic plasticity, reversed both basal turnover and activity-mediated spine stabilization. It also restored defective long-term potentiation mechanisms in slices and improved reversal learning in Fmr1-KO mice. These results suggest that modulation of PI3K signaling could contribute to improve the cognitive deficits associated with FXS.[1] In addition to MCPG, our study suggests that another interesting molecule to reverse phenotypes in Fmr1-KO mice is BpV, a PTEN inhibitor. Application of BpV enhances PI3K activity, a signaling pathway activated by many surface receptors including BDNF that also inhibits LTD (Jurado et al., 2010) and contributes to LTP signaling (Tang et al., 2002). Consistent with this, our experiments show that BpV could restore activity-dependent spine stabilization. These results thus suggest that in addition to an exaggerated LTD type of signaling, another defect present in Fmr1-KO mice could involve defective LTP mechanisms. Our LTP experiments support this interpretation and are in line with those of several other studies (Lauterborn et al., 2007; Meredith et al., 2007; Hu et al., 2008; but see also Krueger & Bear, 2011). A question, however, remains regarding the specific role of PI3K signaling in FXS. Two recent studies showed that PI3K activity and AKT phosphorylation are already upregulated in Fmr1-KO mice (Gross et al., 2010; Sharma et al., 2010), a result at variance with another study (Hu et al., 2008). It should be noted, however, that the PI3K-AKT signaling pathway is downstream of many cell surface receptors and implicated in many interactions with other systems, possibly through differential regulations of AKT phosphorylation sites (Hemmings & Restuccia, 2012). Although a precise understanding of the complex signaling mechanisms implicated in PI3K signaling is beyond the scope of this study, our data clearly show that BpV treatment enhanced AKT phosphorylation on the S473 site both under in vitro and in vivo conditions, and was able not only to reverse the deficits in spine dynamics observed in Fmr1-KO mice, but also to increase LTP and improve Fmr1-KO mice behavior in a Morris water maze learning task. Consistent with these results, PI3K signaling has been shown to contribute to LTP mechanisms (Tang et al., 2002), to be involved in BDNF signaling, which also rescued LTP in Fmr1-KO mice (Lauterborn et al., 2007), to regulate synaptogenesis and spinogenesis in hippocampal neurons (Jaworski et al., 2005; Cuesto et al., 2011), and more importantly to be also deficient in a mouse model of Angelman syndrome, another neurodevelopment disorder with severe cognitive deficits and autistic traits (Cao et al., 2013). It might therefore represent an interesting target for reversing cognitive deficits.[1] NMDA receptors play a key role in spatial and contextual learning and memory (see Squire, 1992). Our results suggest that mGlu receptors may have only a modulatory or secondary role. A recent study found that intra-hippocampal injections of MCPG did not affect working memory, but co-application of MCPG and a NMDA antagonist did (Ohno and Watanabe, 1996). A modulatory rather than a central role for mGlu receptors is also suggested by studies using LTP as a model for learning and memory. While NMDA receptor antagonists clearly block induction of LTP both in vitro (Collingridge et al., 1983) and in vivo (Morris et al., 1986; Abraham and Mason, 1988), the mGlu receptor antagonist MCPG has been reported to have mixed effects, sometimes depending on the technique used (see BenAri and Aniksztejn, 1995). Bashir et al. (1993)reported that MCPG blocked induction of LTP in hippocampal slices, but this result has not been confirmed by other investigators (Chinestra et al., 1993; Manzoni et al., 1994; Selig et al., 1995). Similarly, Riedel and Reymann (1993)found that MCPG blocked induction of LTP in vivo, but others (Bordi and Ugolini, 1995; BenAri and Aniksztejn, 1995) found no effect. In general, there is a good correlation between the effects of an antagonist compound on LTP and its effects on spatial memory tests (but see Saucier and Cain, 1995and Bannerman et al., 1995for further discussion). Because the present data show that MCPG causes some impairment on a spatial learning test but not in another, LTP might be expected to be only partially (Richter-Levin et al., 1994) or minimally affected by MCPG. Future work is needed with more potent and selective mGlu receptor antagonists to shed light on the relationship between different types of learning and the mechanism of LTP. In conclusion, our results show that the mGlu receptor antagonist MCPG disrupts the performance of rats in the spatial version of the water maze, but only using a high concentration of the drug and not in a cued version of the water maze, excluding an effect of the drug on sensory/perceptual faculties. Performance in a context-specific associative learning task was not affected by MCPG, but the NMDA blocker MK801 severely disrupted this performance as it did in the spatial version of the water maze. Future work might utilize these two behavioral paradigms to discriminate between the effects of ionotropic and metabotropic glutamate receptors in learning and memory.[2] The purpose of the study was to investigate the role of metabotropic glutamate receptors (mGluR) in the ontogeny of amphetamine-induced behavioral sensitization. Eleven-day-old rat pups were given five daily bilateral infusions of the mGluR antagonist, (RS)-methyl-4-carboxyphenylglycine (MCPG) followed by a systemic injection of amphetamine and locomotor activity was measured. It was hypothesized that rats receving amphetamine pretreatment and an amphetamine challenge would exhibit a significant increase in activity, indicating short-term behavioral sensitization. As predicted, repeated amphetamine administration during the pretreatment phase produced progressively enhanced locomotor activity, indicating the development of behavioral sensitization. The effect of MCPG on locomotor activity appears to be independent from the effects of amphetamine-induced locomotor activity and MCPG pretreatment failed to consistently block the expression of behavioral sensitization in rats pretreated with amphetamine and challenged with amphetamine. This study demonstrated that contrary to previous studies on adult rats, the mGluR system does not appear to consistently mediate the development of amphetamine-induced sensitization in neonatal rats.[3] The equilibrium potential for GABA-A receptor mediated currents (EGABA) in neonatal central neurons is set at a relatively depolarized level, which is suggested to be caused by a low expression of K+/Cl- co-transporter (KCC2) but a relatively high expression of Na+-K+-Cl- cotransporter (NKCC1). Theta-burst stimulation (TBS) in stratum radiatum induces a negative shift in EGABA in juvenile hippocampal CA1 pyramidal neurons. In the current study, the effects of TBS on EGABA in neonatal and juvenile hippocampal CA1 neurons and the underlying mechanisms were examined. Metabotropic glutamate receptors (mGluRs) are suggested to modulate KCC2 and NKCC1 levels in cortical neurons. Therefore, the involvement of mGluRs in the regulation of KCC2 or NKCC1 activity, and thus EGABA, following TBS was also investigated. Whole-cell patch recordings were made from Wistar rat hippocampal CA1 pyramidal neurons, in a slice preparation. In neonates, TBS induces a positive shift in EGABA, which was prevented by NKCC1 antisense but not NKCC1 sense mRNA. (RS)-a-Methyl-4-carboxyphenylglycine (MCPG), a group I and II mGluR antagonist, blocked TBS-induced shifts in both juvenile and neonatal hippocampal neurons. While blockade of mGluR1 or mGluR5 alone could interfere with TBS-induced shifts in EGABA in neonates, only a combined blockade could do the same in juveniles. These results indicate that TBS induces a negative shift in EGABA in juvenile hippocampal neurons but a positive shift in neonatal hippocampal neurons via corresponding changes in KCC2 and NKCC1 expressions, respectively. mGluR activation seems to be necessary for both shifts to occur while the specific receptor subtype involved seems to vary.[4] |
| 分子式 |
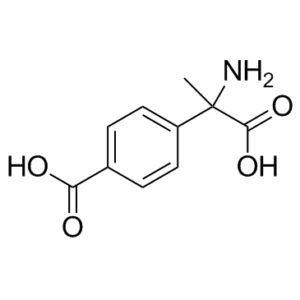
C₁₀H₁₁NO₄
|
|
|---|---|---|
| 分子量 |
209.20
|
|
| 精确质量 |
209.069
|
|
| 元素分析 |
C, 57.41; H, 5.30; N, 6.70; O, 30.59
|
|
| CAS号 |
146669-29-6
|
|
| 相关CAS号 |
(S)-MCPG; 150145-89-4; 1303994-09-3
|
|
| PubChem CID |
1222
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.39g/cm3
|
|
| 沸点 |
230 °C17 mm Hg(lit.)
|
|
| 熔点 |
95-98 °C(lit.)
|
|
| 闪点 |
221-223°C/10mm
|
|
| 蒸汽压 |
5.44E-08mmHg at 25°C
|
|
| LogP |
1.343
|
|
| tPSA |
100.62
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
3
|
|
| 重原子数目 |
15
|
|
| 分子复杂度/Complexity |
271
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
CC(C(O)=O)(N)C1=CC=C(C(O)=O)C=C1
|
|
| InChi Key |
DNCAZYRLRMTVSF-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C10H11NO4/c1-10(11,9(14)15)7-4-2-6(3-5-7)8(12)13/h2-5H,11H2,1H3,(H,12,13)(H,14,15)
|
|
| 化学名 |
4-(1-amino-1-carboxyethyl)benzoic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.7801 mL | 23.9006 mL | 47.8011 mL | |
| 5 mM | 0.9560 mL | 4.7801 mL | 9.5602 mL | |
| 10 mM | 0.4780 mL | 2.3901 mL | 4.7801 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。