| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Cyclooxygenase-2 (COX-2) (IC50 = 12.7 μM); Cyclooxygenase-1 (COX-1) (IC50 = 71.5 μM) [1]
- Lymphocytes, macrophages, T/B cells [3] |
|---|---|
| 体外研究 (In Vitro) |
狼疮的许多有趣的生物学特性已被证明,包括对内分泌和心血管系统的影响、抗血栓、抗癌、抗炎和镇痛、抗肥胖和体温调节以及血管舒张活性[2]。吴茱萸碱的 IC50 分别为 0.28 μM 和 8.7 μM,以浓度依赖性方式抑制 BMMC 中 PGD2 合成的 COX-2 和 COX-1 依赖相。它以剂量依赖性方式抑制 COX-2 转染的 HEK293 细胞将外源花生四烯酸转化为 PGE2 的 COX-2 依赖性转化 [1]。
吴茱萸碱(Rutaecarpine, Rutecarpine)对COX-2具有选择性抑制活性,抑制COX-2介导的前列腺素E2(PGE2)生成,IC50为12.7 μM,对COX-1的抑制作用较弱(IC50 = 71.5 μM),COX-2/COX-1选择性比率约为5.6[1] - 吴茱萸碱(Rutaecarpine, Rutecarpine)具有体外免疫抑制活性:以剂量依赖性方式(有效浓度范围10-80 μM)抑制刀豆蛋白A(Con A)诱导的T淋巴细胞增殖和脂多糖(LPS)诱导的B淋巴细胞增殖,同时减少活化淋巴细胞中促炎细胞因子(IL-2、IFN-γ、TNF-α)的产生[3] - 吴茱萸碱(Rutaecarpine, Rutecarpine)在无细胞体系中具有抗氧化活性,可清除羟自由基和超氧阴离子,清除率在浓度高达100 μM时呈剂量依赖性升高[2] |
| 体内研究 (In Vivo) |
通过腹腔注射吴茱萸碱治疗L-角叉菜胶引起的大鼠爪水肿,显示出体内抗炎作用[1]。卢平诱导抗体形成细胞数量呈剂量依赖性减少以及脾脏重量下降。此外,服用吴茱萸碱的动物表现出脾脏细胞结构减少以及脾脏总T细胞、CD4+细胞、CD8+细胞和B细胞减少。 IL-2、干扰素和IL-10 mRNA表达均被吴茱萸碱治疗显着抑制。给小鼠服用番石榴后,CD4+IL-2+细胞的数量显着减少[3]。
在角叉菜胶诱导的大鼠足肿胀炎症模型中,口服吴茱萸碱(Rutaecarpine, Rutecarpine)(50 mg/kg、100 mg/kg)以剂量依赖性方式显著减轻足肿胀,给药后4小时抑制率分别约为35%和58%,该抗炎效应与炎症组织中PGE2水平降低相关[1] - 在雌性BALB/c小鼠中,口服吴茱萸碱(Rutaecarpine, Rutecarpine)(20 mg/kg、40 mg/kg,每日1次,持续14天)发挥免疫抑制作用:降低绵羊红细胞(SRBC)免疫小鼠的血清抗体水平(IgG、IgM),抑制SRBC诱导的迟发型超敏反应(DTH),减少脾脏淋巴细胞数量[3] - 吴茱萸碱(Rutaecarpine, Rutecarpine)(30 mg/kg,口服灌胃,持续7天)减少LPS诱导的小鼠肺组织炎症细胞浸润,降低血清TNF-α和IL-6水平[2] |
| 酶活实验 |
COX-1/COX-2活性实验:将纯化的重组COX-1和COX-2酶与花生四烯酸(底物)及不同浓度的吴茱萸碱(Rutaecarpine, Rutecarpine)在37°C下孵育30分钟。ELISA法检测PGE2生成量,根据对PGE2合成的抑制效应计算IC50值[1]
- 抗氧化实验:构建羟自由基/超氧阴离子生成体系,加入0-100 μM 吴茱萸碱(Rutaecarpine, Rutecarpine),通过特异性比色或荧光探针检测剩余自由基,量化自由基清除率[2] |
| 细胞实验 |
淋巴细胞增殖实验:分离小鼠脾脏淋巴细胞,在含10-80 μM 吴茱萸碱(Rutaecarpine, Rutecarpine)的条件下,用Con A(诱导T细胞)或LPS(诱导B细胞)培养72小时。MTT法检测细胞增殖,计算抑制率[3]
- 细胞因子检测实验:活化淋巴细胞(经Con A/LPS + 吴茱萸碱(Rutaecarpine, Rutecarpine)处理)培养48小时后,收集上清液,ELISA法检测IL-2、IFN-γ、TNF-α水平[3] - 炎症细胞实验:RAW264.7巨噬细胞用0-50 μM 吴茱萸碱(Rutaecarpine, Rutecarpine)预处理2小时后,加入LPS刺激。Griess试剂检测一氧化氮(NO)生成,ELISA法检测PGE2水平[2] |
| 动物实验 |
Anti-inflammatory model: Rats were randomly divided into control and Rutaecarpine (Rutecarpine) treatment groups. Rutaecarpine (Rutecarpine) was dissolved in 0.5% carboxymethylcellulose sodium and administered by oral gavage at 50 mg/kg or 100 mg/kg 1 hour before carrageenan injection. Paw volume was measured at 1, 2, 4, and 6 hours post-carrageenan injection [1]
- Immunosuppressive model: Female BALB/c mice were immunized with SRBC via intraperitoneal injection. Rutaecarpine (Rutecarpine) was dissolved in corn oil and administered by oral gavage at 20 mg/kg or 40 mg/kg once daily for 14 days (starting from the day of immunization). Serum antibody levels were detected by hemagglutination assay, and DTH reactions were evaluated by footpad swelling measurement [3] - LPS-induced inflammation model: Mice were treated with Rutaecarpine (Rutecarpine) (30 mg/kg, oral gavage) once daily for 7 days. On the 7th day, LPS was injected intraperitoneally, and mice were sacrificed 6 hours later to collect serum and lung tissues for inflammatory index detection [2] |
| 药代性质 (ADME/PK) |
Metabolism / Metabolites
Rutaecarpine has known human metabolites that include 3-Hydroxyrutaecarpine, 6-Hydroxy-3,13,21-triazapentacyclo[11.8.0.02,10.04,9.015,20]henicosa-1(21),2(10),4(9),5,7,15,17,19-octaen-14-one, 5-Hydroxy-3,13,21-triazapentacyclo[11.8.0.02,10.04,9.015,20]henicosa-1(21),2(10),4(9),5,7,15,17,19-octaen-14-one, 8-Hydroxy-3,13,21-triazapentacyclo[11.8.0.02,10.04,9.015,20]henicosa-1(21),2(10),4(9),5,7,15,17,19-octaen-14-one, 11-hydroxy-3,13,21-triazapentacyclo[11.8.0.02,10.04,9.015,20]henicosa-1(21),2(10),4,6,8,15,17,19-octaen-14-one, and 7-Hydroxy-3,13,21-triazapentacyclo[11.8.0.02,10.04,9.015,20]henicosa-1(21),2(10),4(9),5,7,15,17,19-octaen-14-one. Rutaecarpine (Rutecarpine) showed poor oral bioavailability (~3.2%) in rats due to extensive first-pass metabolism in the liver [2] - It was rapidly metabolized in the liver via cytochrome P450 enzymes (CYP3A4, CYP2C9), producing hydroxylated metabolites [2] - The plasma half-life (t1/2) of Rutaecarpine (Rutecarpine) in rats after intravenous administration (5 mg/kg) was ~1.8 hours [2] - It distributed widely in tissues, with higher concentrations in the liver, kidney, and lung, and lower concentrations in the brain [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
In vivo, oral administration of Rutaecarpine (Rutecarpine) at doses up to 100 mg/kg for 14 days did not cause significant changes in mouse body weight, organ index, or serum ALT/AST/creatinine levels [3]
- The acute oral LD50 of Rutaecarpine (Rutecarpine) in mice was > 2000 mg/kg, indicating low acute toxicity [2] - No obvious cytotoxicity was observed in normal hepatocytes (LO2) and spleen lymphocytes at concentrations up to 100 μM in vitro [2][3] |
| 参考文献 | |
| 其他信息 |
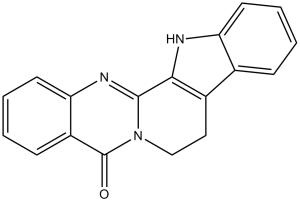
Rutecarpine is a member of beta-carbolines.
Rutaecarpine has been reported in Tetradium ruticarpum, Zanthoxylum wutaiense, and other organisms with data available. Rutaecarpine (Rutecarpine) is a quinazoline alkaloid isolated from the dried fruits of Evodia rutaecarpa (Juss.) Benth. [1][2] - Its anti-inflammatory mechanism is mainly mediated by selective inhibition of COX-2, reducing prostaglandin synthesis [1] - The immunosuppressive effect is associated with inhibition of T/B lymphocyte proliferation and pro-inflammatory cytokine production, suggesting potential application in autoimmune diseases [3] |
| 分子式 |
C18H13N3O
|
|
|---|---|---|
| 分子量 |
287.32
|
|
| 精确质量 |
287.105
|
|
| CAS号 |
84-26-4
|
|
| 相关CAS号 |
|
|
| PubChem CID |
65752
|
|
| 外观&性状 |
Light yellow to yellow solid powder
|
|
| 密度 |
1.5±0.1 g/cm3
|
|
| 沸点 |
550.1±60.0 °C at 760 mmHg
|
|
| 熔点 |
259.5 - 260ºC
|
|
| 闪点 |
286.5±32.9 °C
|
|
| 蒸汽压 |
0.0±1.5 mmHg at 25°C
|
|
| 折射率 |
1.792
|
|
| LogP |
2.03
|
|
| tPSA |
50.68
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
2
|
|
| 可旋转键数目(RBC) |
0
|
|
| 重原子数目 |
22
|
|
| 分子复杂度/Complexity |
517
|
|
| 定义原子立体中心数目 |
0
|
|
| InChi Key |
ACVGWSKVRYFWRP-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C18H13N3O/c22-18-13-6-2-4-8-15(13)20-17-16-12(9-10-21(17)18)11-5-1-3-7-14(11)19-16/h1-8,19H,9-10H2
|
|
| 化学名 |
8,13-dihydro-indolo[2,3:3,4]pyrido[2,1-b]quinazolin-5(7H)-one
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.4804 mL | 17.4022 mL | 34.8044 mL | |
| 5 mM | 0.6961 mL | 3.4804 mL | 6.9609 mL | |
| 10 mM | 0.3480 mL | 1.7402 mL | 3.4804 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。